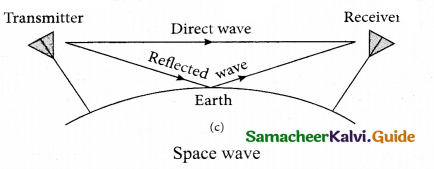
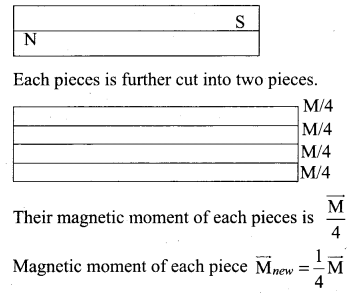
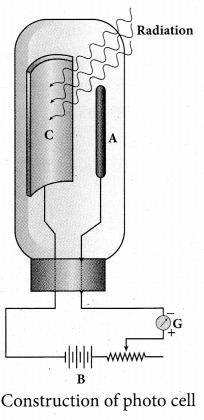
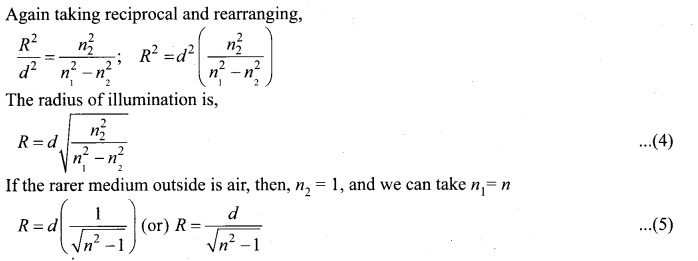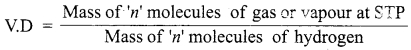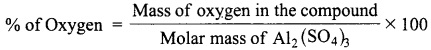Students can download 6th Science Term 2 Chapter 7 Parts of Computer Questions and Answers, Notes, Samacheer Kalvi 6th Science Guide Pdf helps you to revise the complete Tamilnadu State Board New Syllabus, helps students complete homework assignments and to score high marks in board exams.
Tamilnadu Samacheer Kalvi 6th Science Solutions Term 2 Chapter 7 Parts of Computer
Samacheer Kalvi 6th Science Parts of Computer Text Book Back Questions and Answers
I. Choose the correct answer:
Question 1.
Which one of the following is an output device?
(a) Mouse
(b) Keyboard
(c) Speaker
(d) Pen drive
Answer:
(c) Speaker
Question 2.
Name the cable that connects CPU to the Monitor
(a) Ethernet
(b) VGA cable
(c) HDMI
(d) USB
Answer:
(b) VGA cable
![]()
Question 3.
Which one of the following is an input device?
(a) Speaker
(b) Keyboard
(c) Monitor
(d) Printer
Answer:
(b) Keyboard
Question 4.
Which one of the following is an example for wireless connections?
(a) Wi-Fi
(b) Electric wires
(c) VGA
(d) USB
Answer:
(a) Wi-Fi
Question 5.
Pen drive is ……… device.
(a) Output
(b) Input
(c) Storage
(d) Connecting cable
Answer:
(c) Storage
![]()
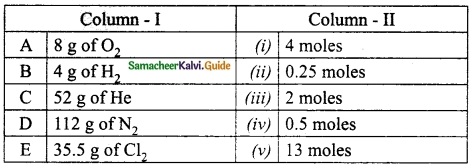
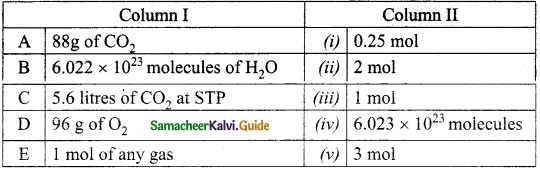
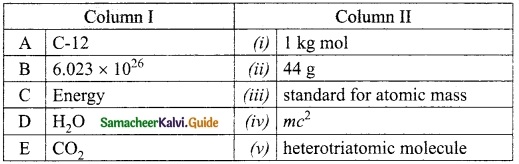
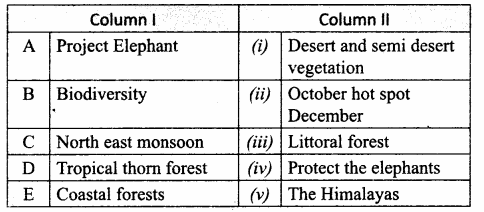
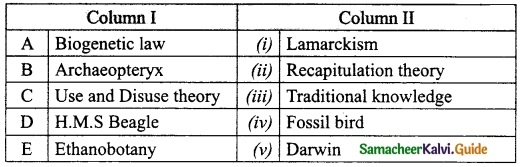
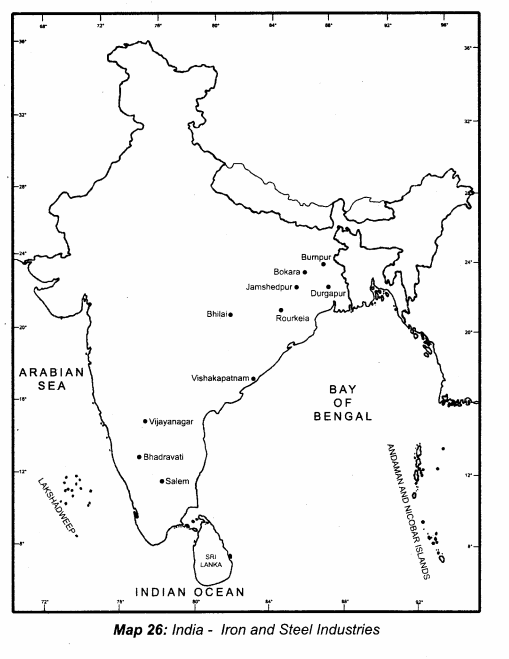
II. Match the following
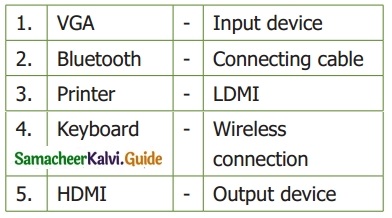
Answer:
1. – Connecting Cable
2. – wireless connection
3. – Output device
4. – Input device
5. – LDMI
III. Give Short Answer:
Question 1.
Name the parts of a computer.
Answer:
The computer has three parts like,
1. Input unit
2. Central Processing Unit
3. Output
![]()
![]()
Question 2.
Answer:
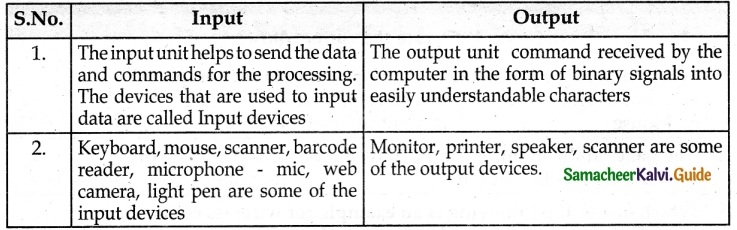
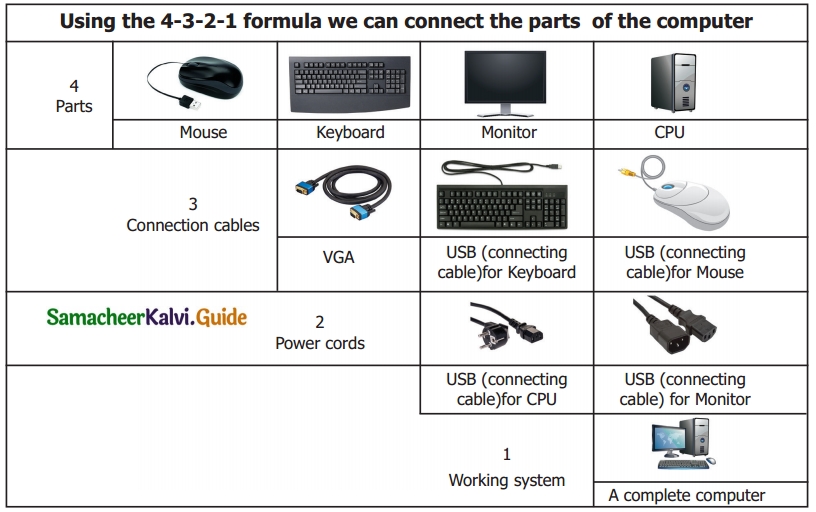
Activity
Look at the magic of connecting cables to desktop computer with 4,3,2,1 formula, start from 4 proceed till 1. Now your computer is ready to use.
By connecting the various parts of a computer we can assemble a computer. For the construction activity, students have to use 4-3-2-1 formula.
A system consist of mouse, key board, monitor, CPU, power cables, and connecting cables Students has to connect the four parts of a computer in row 4, using the cables in row 3, through the power cables in row 2 to construct a system.
![]()
Samacheer Kalvi 6th Science Parts of Computer Additional Important Questions and Answers
I. Choose the correct Answer:
Question 1.
Which one of the following is not important part of computer?
(a) Input device
(b) Output device
(c) Mouse
(d) Central Processing Unit
Answer:
(c) Mouse
Question 2.
The page on the monitor can be moved up and down using the _______
(a) Right button
(b) Scroll ball
(c) Left button
(d) Number key
Answer:
(b) Scroll ball
![]()
Question 3.
Which one of the following is an example for wireless connection.
(a) USB
(b) power cord
(c) HDMI
(d) wi-fi
Answer:
(d) wi-fi
Question 4.
The data is measured in units which is called as _______
(a) micron
(b) meter
(c) millimeter
(d) Bit
Answer:
(d) Bit
II. Fill in the Blanks
- ………, ……….. plays a role of data in computer.
- …….. controls the functions of all parts of the computer.
- Memory unit divided into ………. types.
- The data is measured in units which is called as ………..
- Mouse is connected with the computer using ………..
Answer:
- Numbers and alphabets
- control unit
- 2
- Bit
- USB cord or cable
![]()
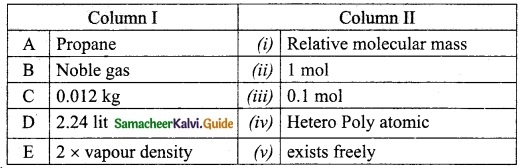
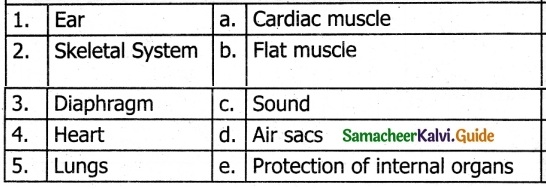
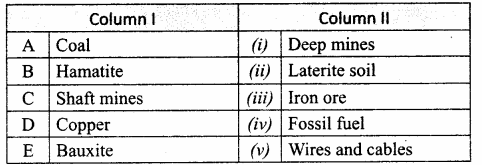
III. Match the following
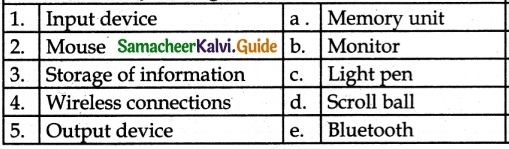
Answer:
1. – c
2. – d
3. – a
4. – e
5. – b
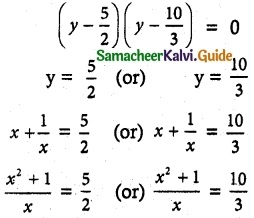
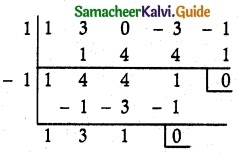
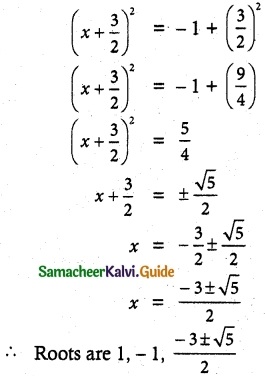
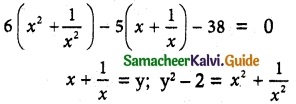

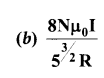
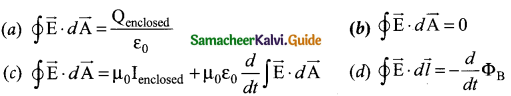
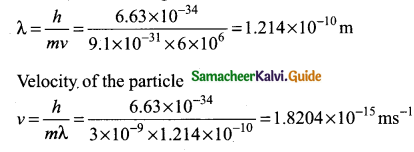

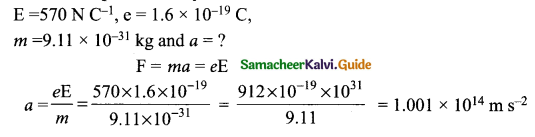
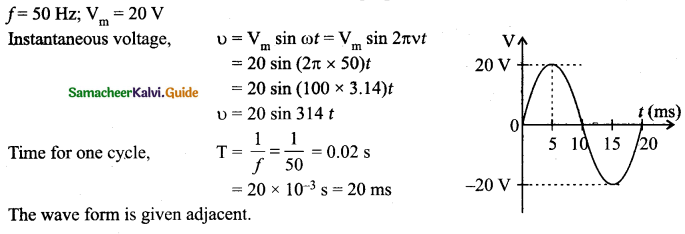
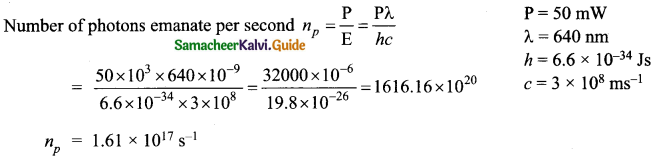
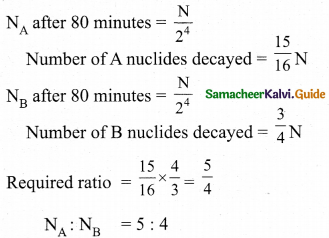
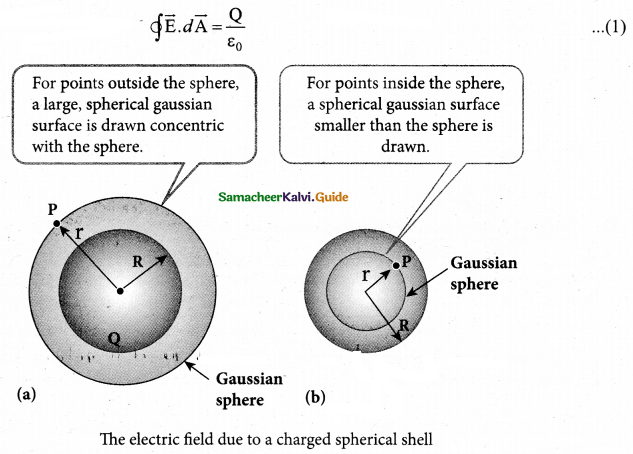
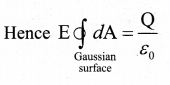
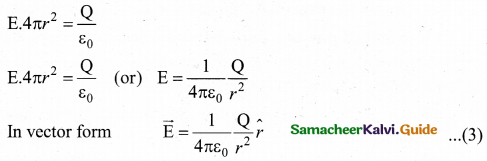
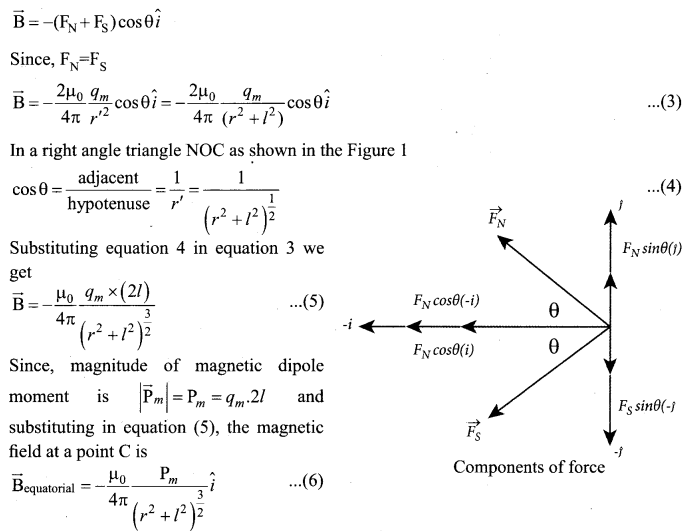
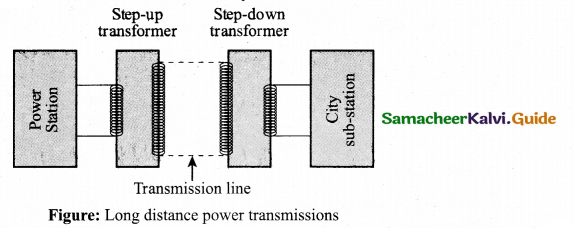
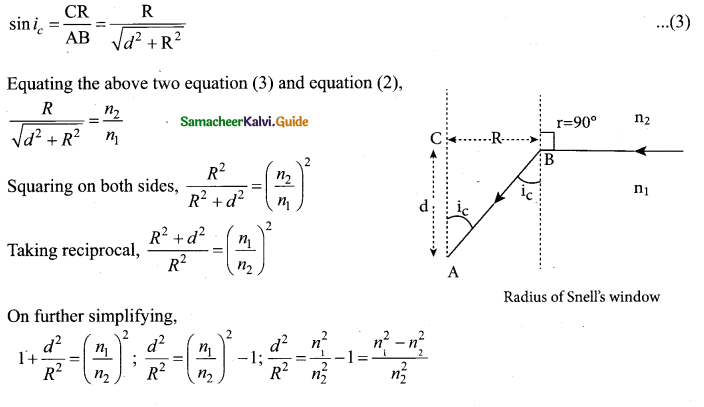
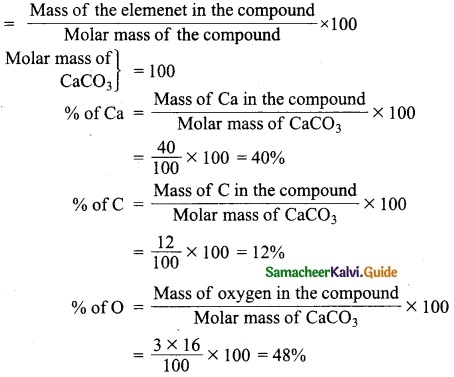
 = total area of Gaussian surface = 4πr2 Substituting this value in equation (2)
= total area of Gaussian surface = 4πr2 Substituting this value in equation (2)