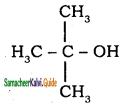
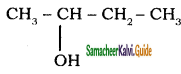
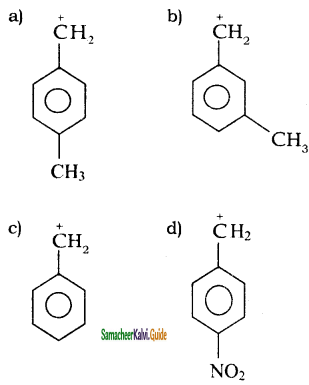
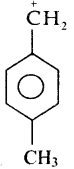
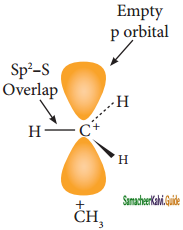
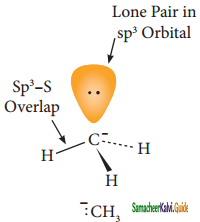
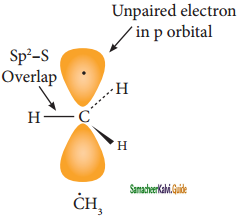
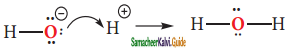
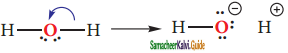
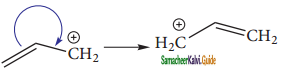
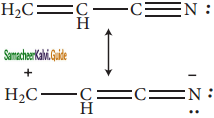
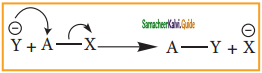
Subject Matter Experts at SamacheerKalvi.Guide have created Tamil Nadu State Board Samacheer Kalvi 11th Books Answers Solutions Guide Pdf Free Download in English Medium and Tamil Medium are part of Samacheer Kalvi Books Solutions.
Let us look at these TN State Board New Syllabus Samacheer Kalvi 11th Std Guide Pdf of Text Book Back Questions and Answers of Volume 1 and Volume 2, Chapter Wise Important Questions, Study Material, Question Bank, Notes, Formulas and revise our understanding of the subject.
Students can also read Tamil Nadu Samacheer Kalvi 11th Model Question Papers 2020-2021.
Tamilnadu Samacheer Kalvi 11th Guide Text Book Back Answers Solutions Pdf Free Download
TN Samacheer Kalvi 11th Book Back Answers Solutions Guide
- Samacheer Kalvi 11th Maths Guide
- Samacheer Kalvi 11th Physics Guide
- Samacheer Kalvi 11th Chemistry Guide
- Samacheer Kalvi 11th Biology Guide
- Samacheer Kalvi 11th Bio Botany Guide
- Samacheer Kalvi 11th Bio Zoology Guide
- Samacheer Kalvi 11th Tamil Guide
- Samacheer Kalvi 11th English Guide
- Samacheer Kalvi 11th History Guide
- Samacheer Kalvi 11th Commerce Guide
- Samacheer Kalvi 11th Accountancy Guide
- Samacheer Kalvi 11th Economics Guide
- Samacheer Kalvi 11th Business Maths Guide
- Samacheer Kalvi 11th Computer Science Guide
- Samacheer Kalvi 11th Computer Applications Guide
We hope these Tamilnadu State Board Samacheer Kalvi Class 11th Std Books Solutions Answers Guide Pdf Free Download in English Medium and Tamil Medium will help you get through your subjective questions in the exam.
Let us know if you have any concerns regarding TN State Board New Syllabus Samacheer Kalvi 11th Standard Guides Pdf of Text Book Back Questions and Answers of Volume 1 and Volume 2, Chapter Wise Important Questions, Study Material, Question Bank, Notes, Formulas, drop a comment below and we will get back to you as soon as possible.
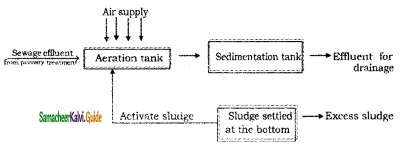
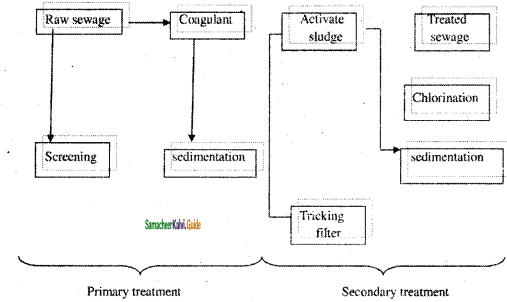
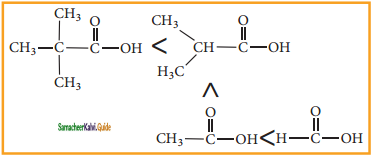
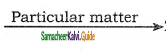 2SO3
2SO3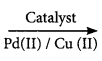 CH3CHO
CH3CHO is
is X is ______.
X is ______.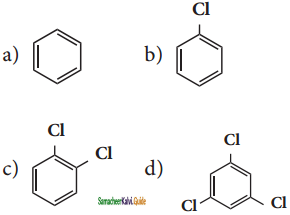
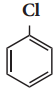




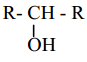


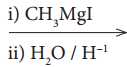 X, X is
X, X is

 C6H5 – C6H5 + 2NaCl
C6H5 – C6H5 + 2NaCl
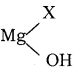


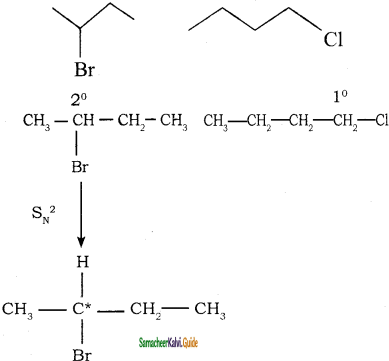
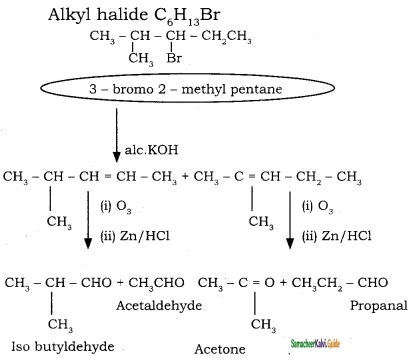
 → 2 – bromo pentane
→ 2 – bromo pentane → 3 – bromo pentane
→ 3 – bromo pentane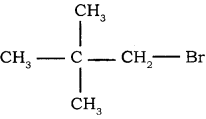 → 1 – bromo 2, 2 – dimethyl propane
→ 1 – bromo 2, 2 – dimethyl propane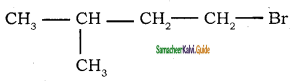 → 1 – bromo 3 – methyl butane
→ 1 – bromo 3 – methyl butane → 2 – bromo 3 – methyl butane
→ 2 – bromo 3 – methyl butane → 2 – bromo 2 – methyl butane
→ 2 – bromo 2 – methyl butane → 1 – bromo 2- methyl butane
→ 1 – bromo 2- methyl butane → (2S) – 1 – bromo 2 – methyl butane
→ (2S) – 1 – bromo 2 – methyl butane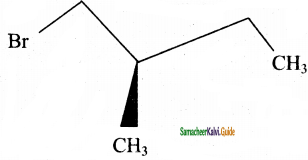 → (2R) – 1 – bromo 2 – methyl butane
→ (2R) – 1 – bromo 2 – methyl butane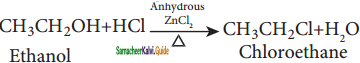



 C6H5NH2 + NH4Cl
C6H5NH2 + NH4Cl


 CCl4 + S2Cl2
CCl4 + S2Cl2







 C6H5 – C6H5 + 2NaCl
C6H5 – C6H5 + 2NaCl 2 HCl + CCl2F2
2 HCl + CCl2F2
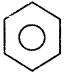 + Cl2
+ Cl2  B, B is
B, B is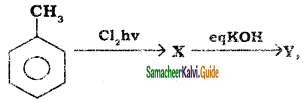 Y is
Y is

 CH3CH2I + NaI
CH3CH2I + NaI CH3CH2Br + CO2 + AgBr
CH3CH2Br + CO2 + AgBr


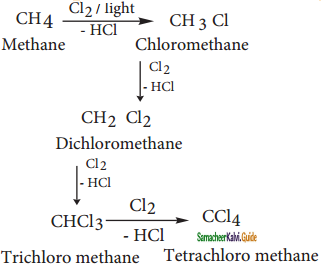
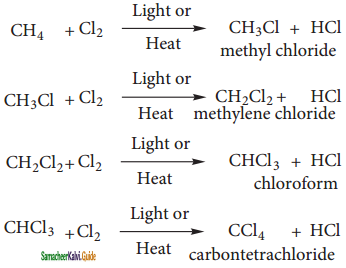
 CH2Cl (Methylene chloride) + HCl
CH2Cl (Methylene chloride) + HCl CH3Cl
CH3Cl 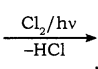 CH2Cl2
CH2Cl2 CHCl3 (chloroform) + HCl
CHCl3 (chloroform) + HCl


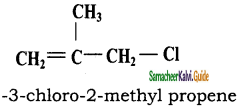
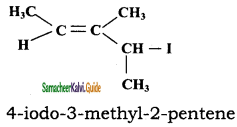




 CH3CH2SH + NaBr
CH3CH2SH + NaBr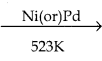 CH3 – CH3 + HBr
CH3 – CH3 + HBr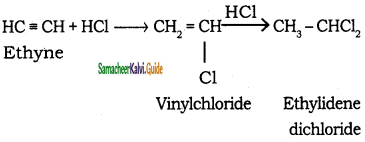



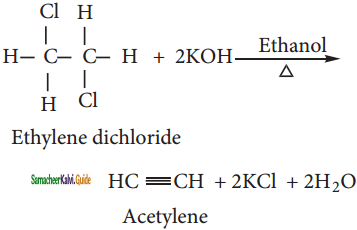
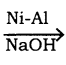 C6H6 + HCl
C6H6 + HCl C6H5MgCl (Phenyl magnesium chloride)
C6H5MgCl (Phenyl magnesium chloride)


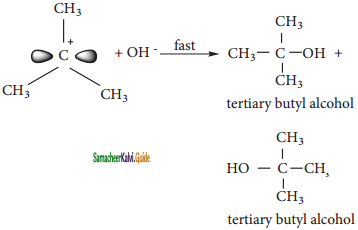

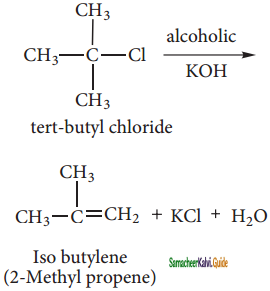


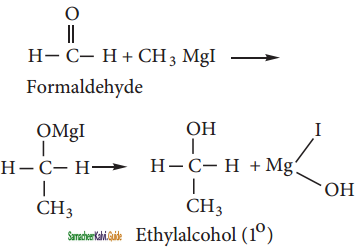
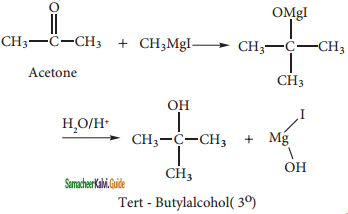





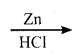 CH2Cl2 (Methylene chloride)+ Cl2
CH2Cl2 (Methylene chloride)+ Cl2

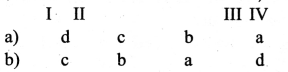
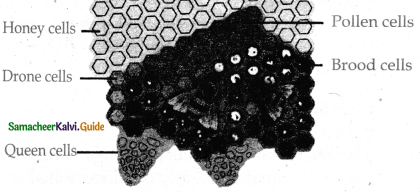
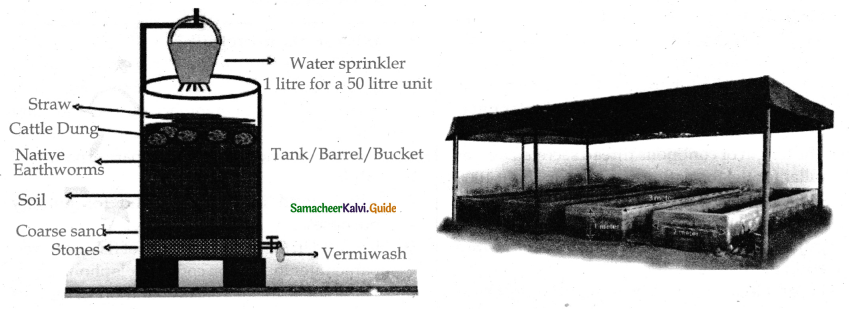
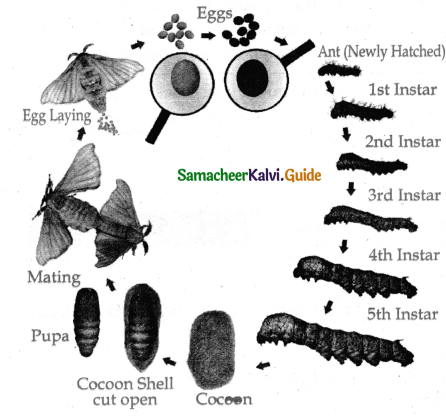


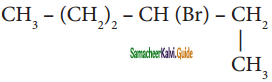
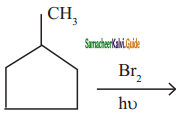
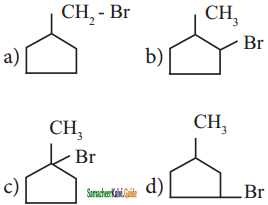
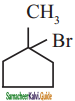
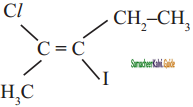
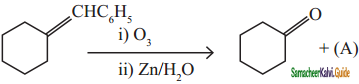
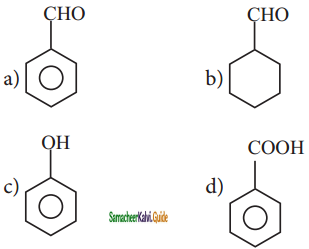
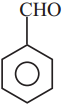
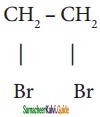
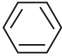
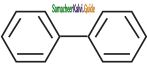
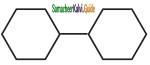
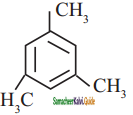
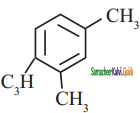
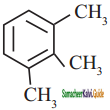
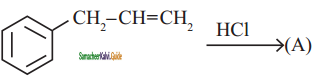 is
is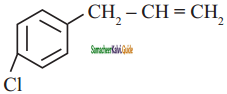
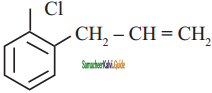
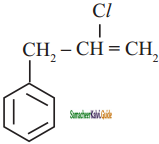
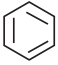
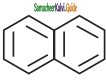
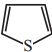
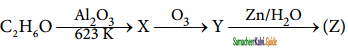
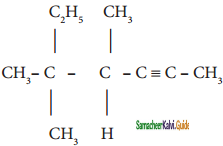
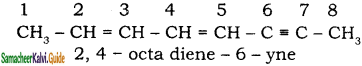
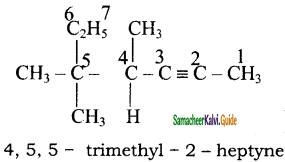
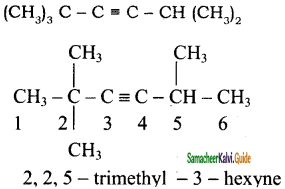
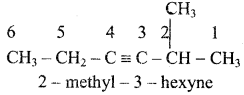
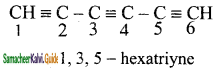
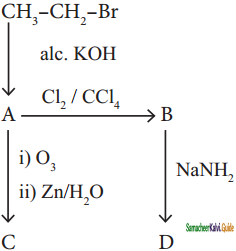
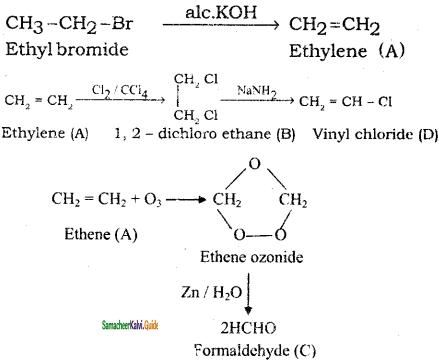
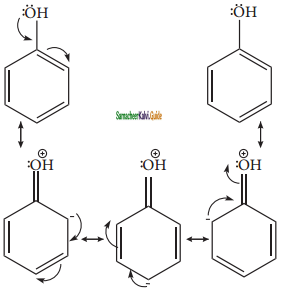
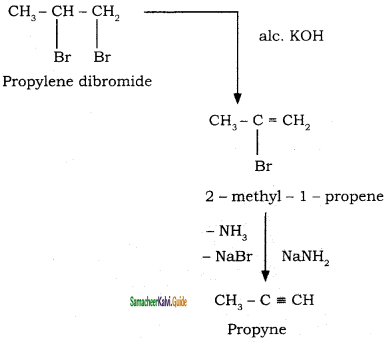
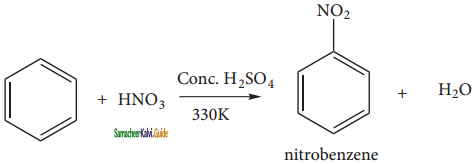
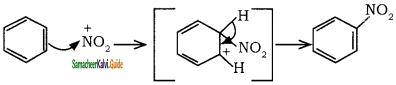
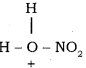 + HSO4–
+ HSO4–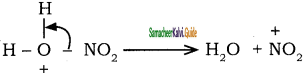
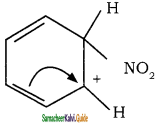
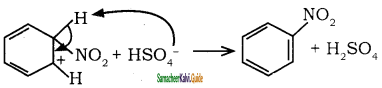
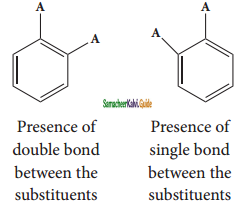
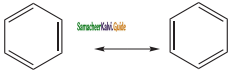
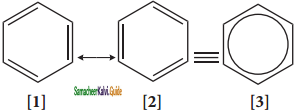
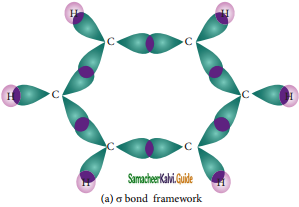
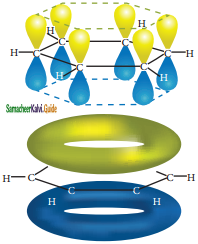
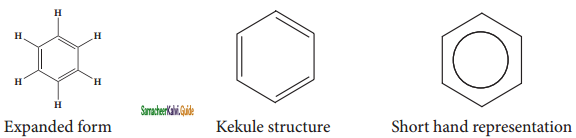
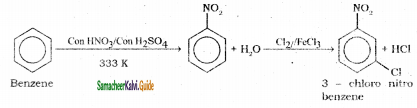
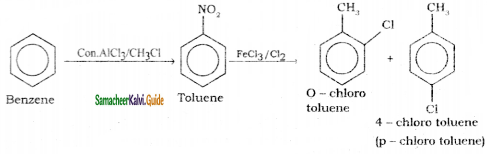
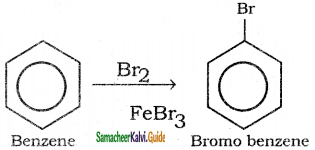
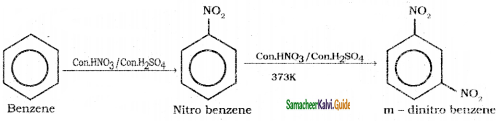
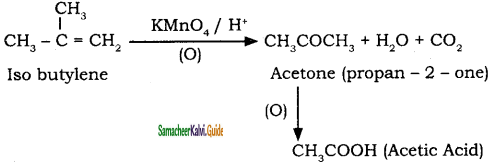
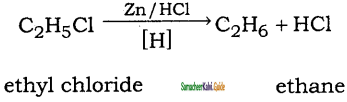
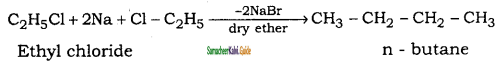
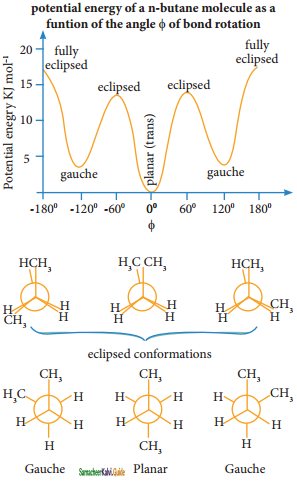
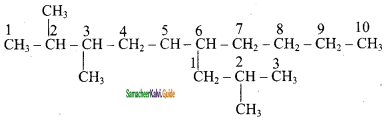
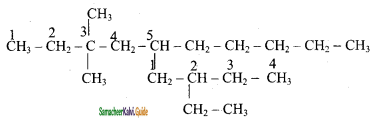
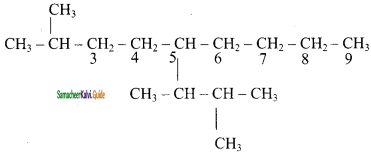
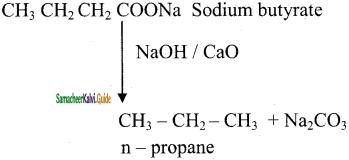
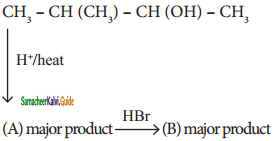
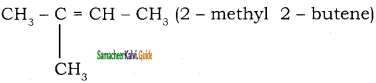
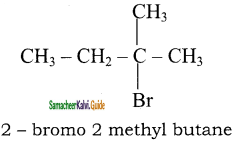
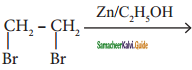
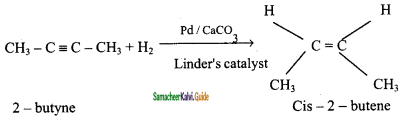
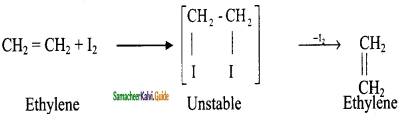
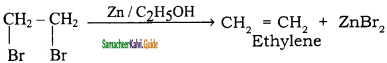
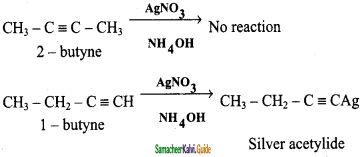
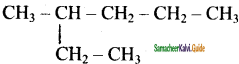
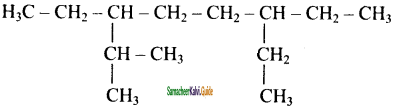
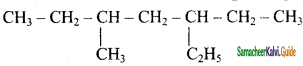
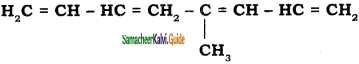
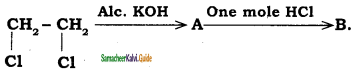 is
is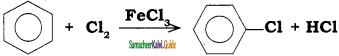

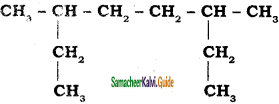
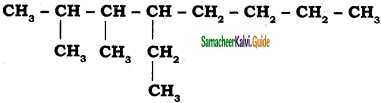
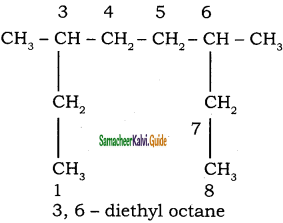
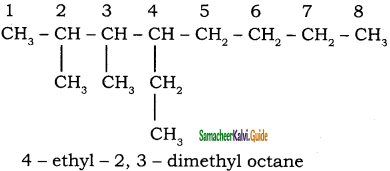
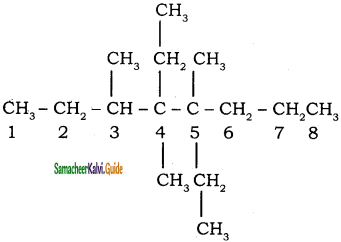
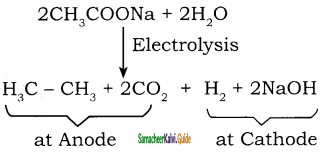
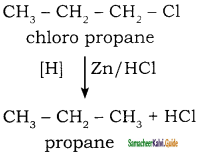
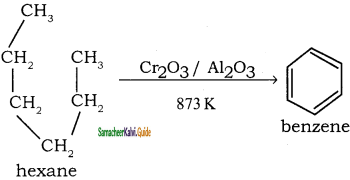
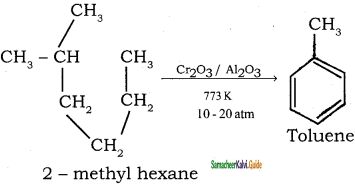
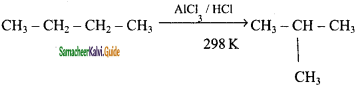
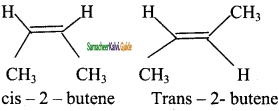
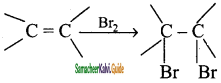
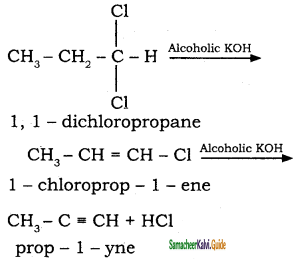
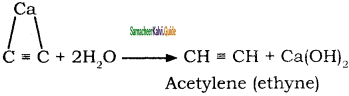
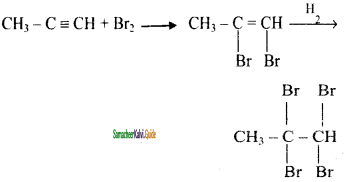
 CH3 – CH3
CH3 – CH3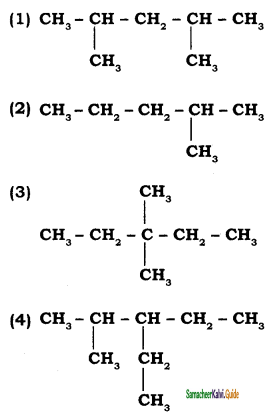
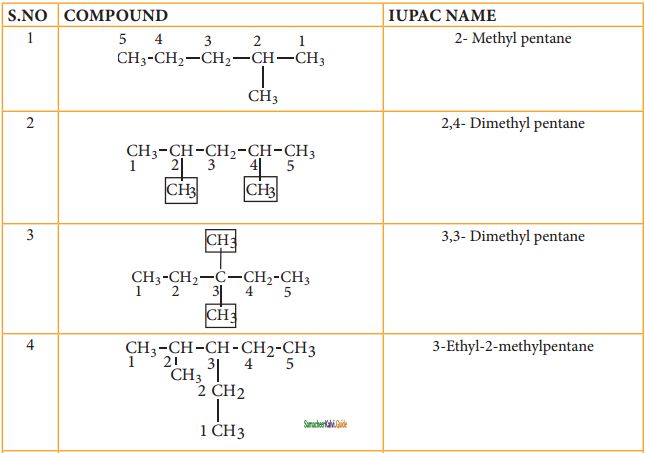
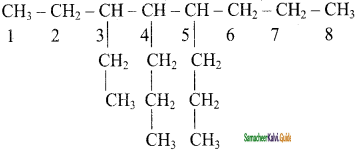
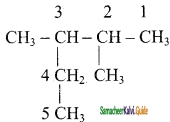
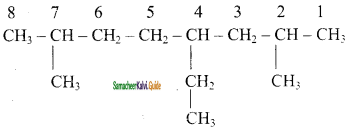
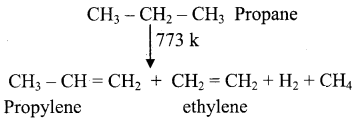
 CH2 = CH2 + 2CH4
CH2 = CH2 + 2CH4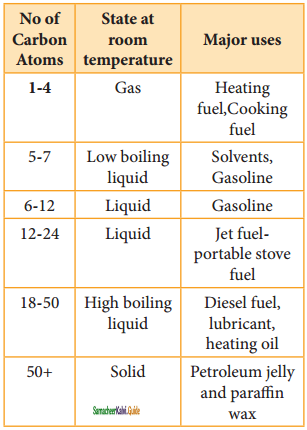
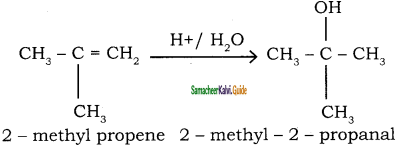
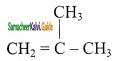 2 – mehyl – 1 – propene
2 – mehyl – 1 – propene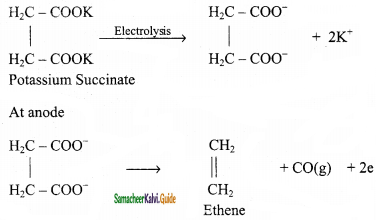
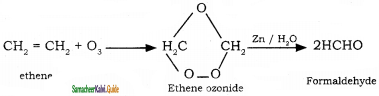
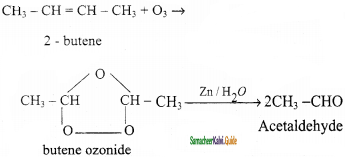
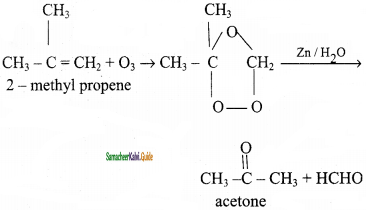
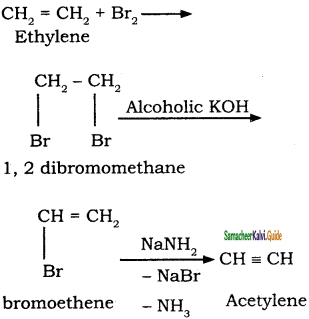
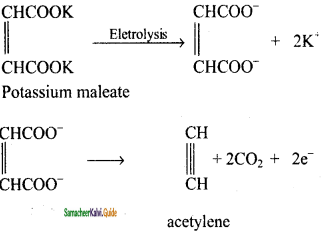
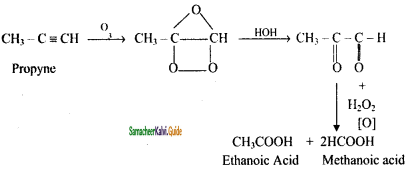
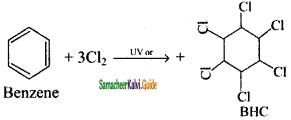
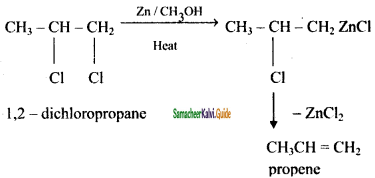
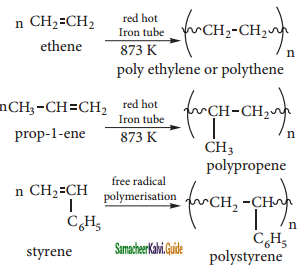
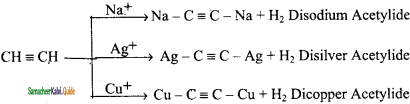
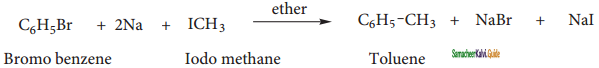

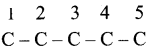
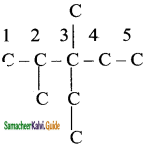
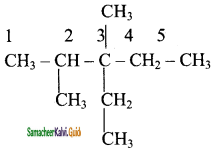
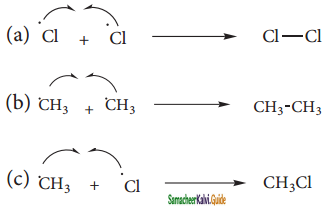
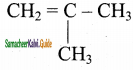
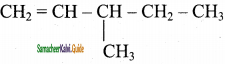
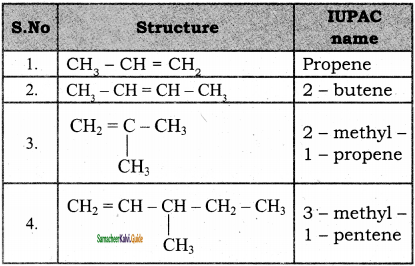
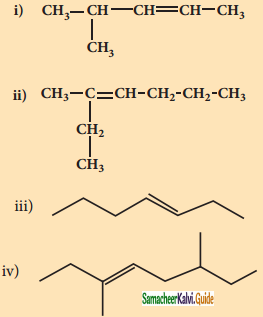
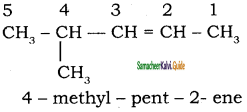
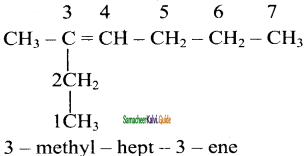
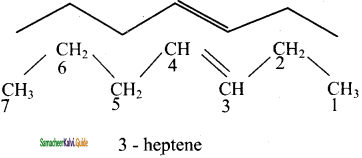
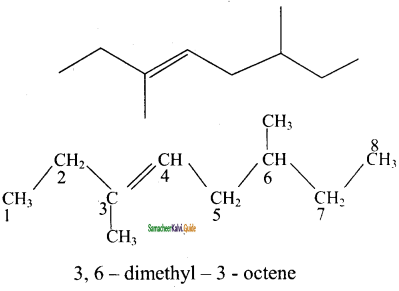
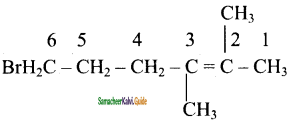
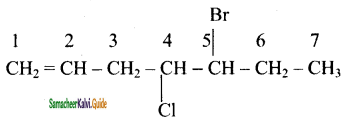
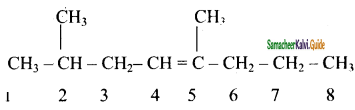
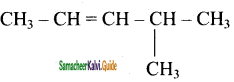
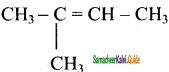
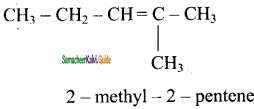
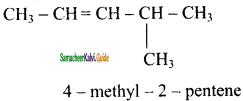
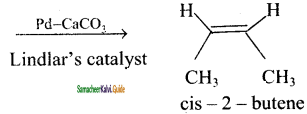
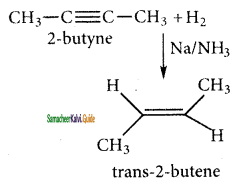
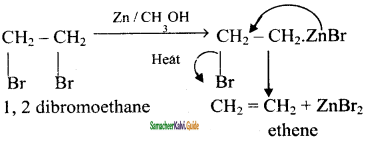
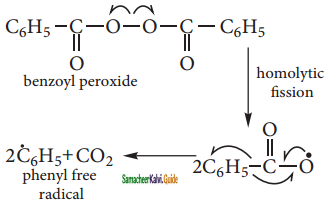
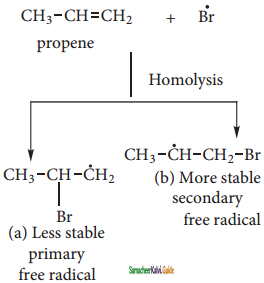
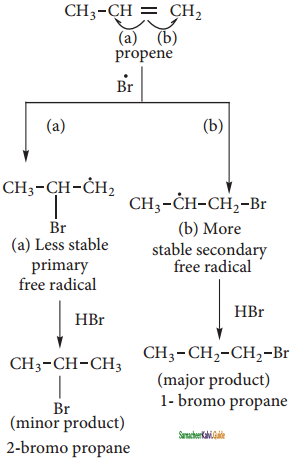
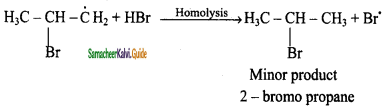
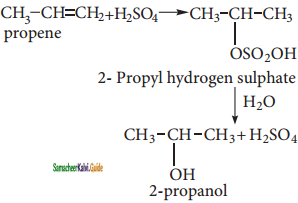

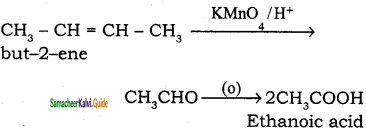
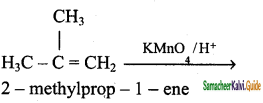
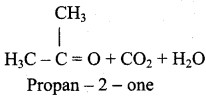
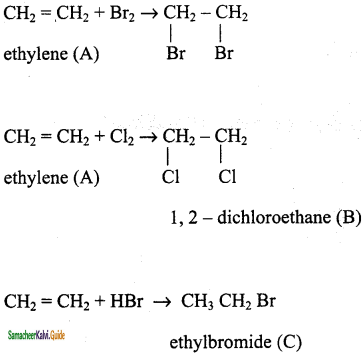
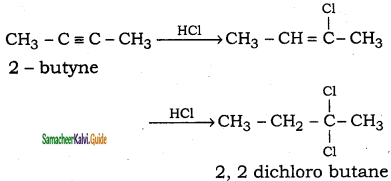
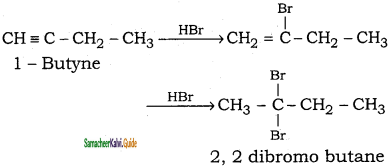
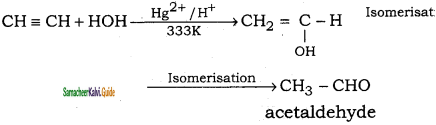
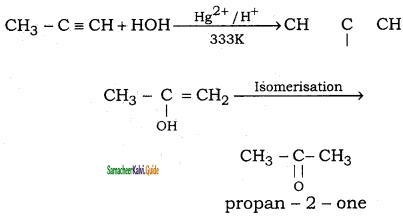
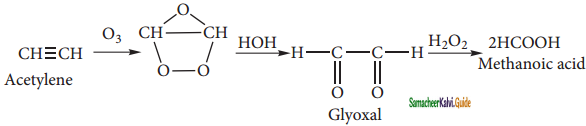
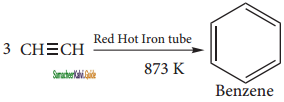

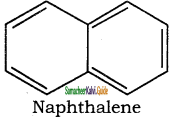
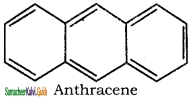
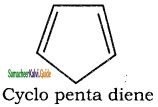
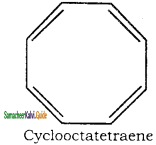
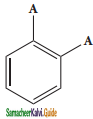
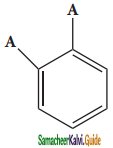
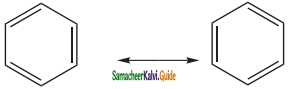
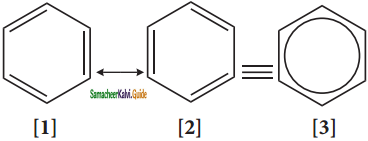
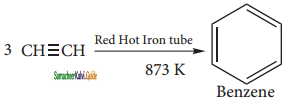
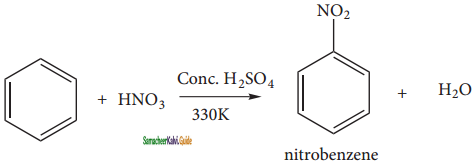
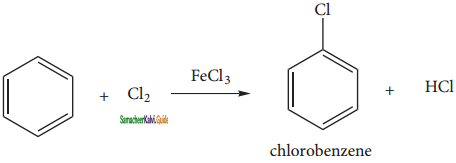
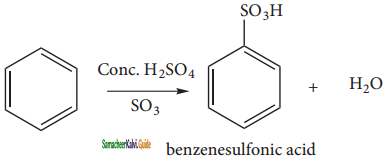
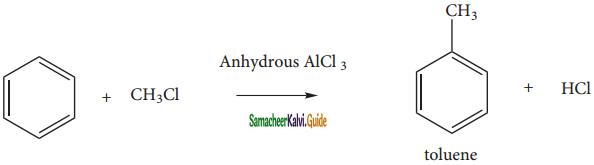
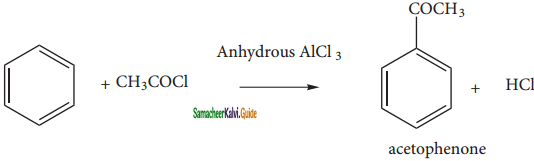
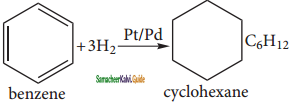
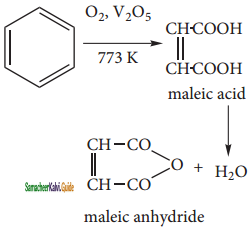
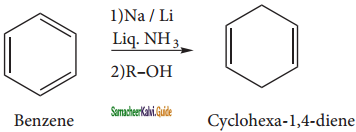
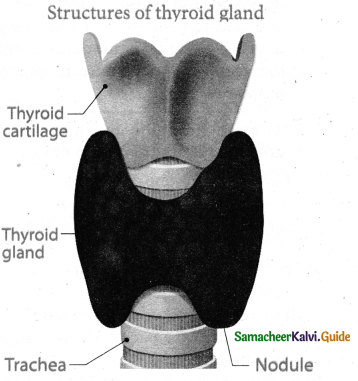
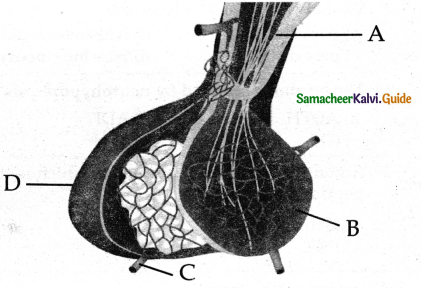
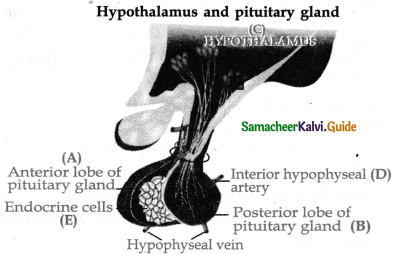
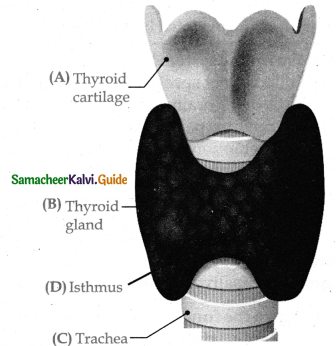
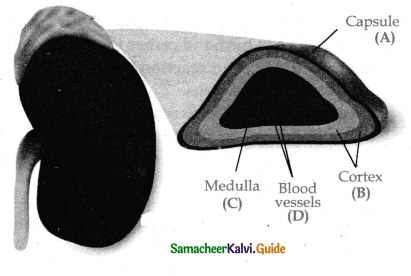
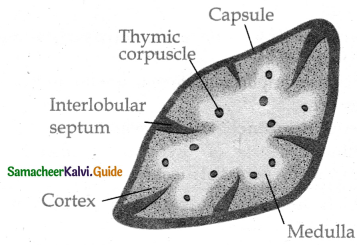
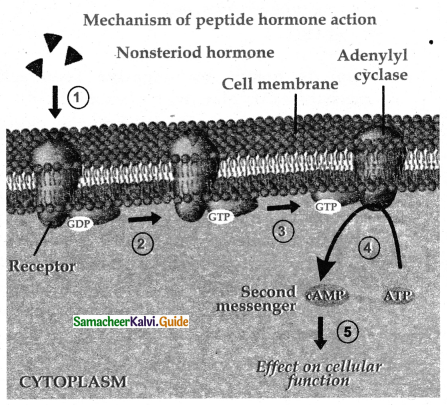
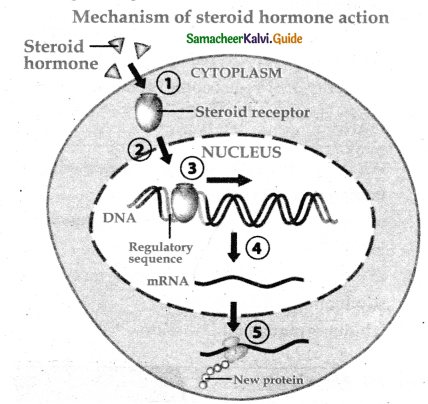
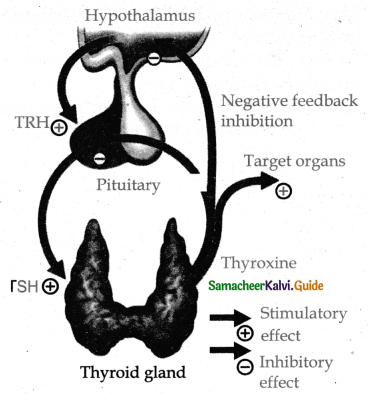

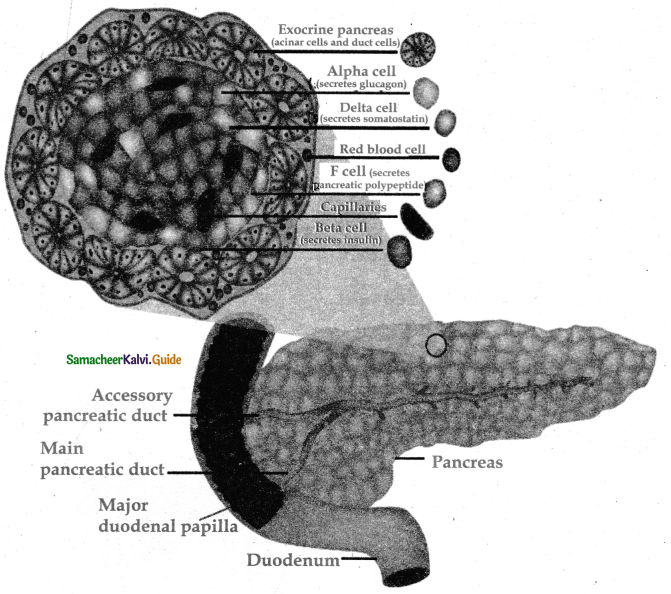
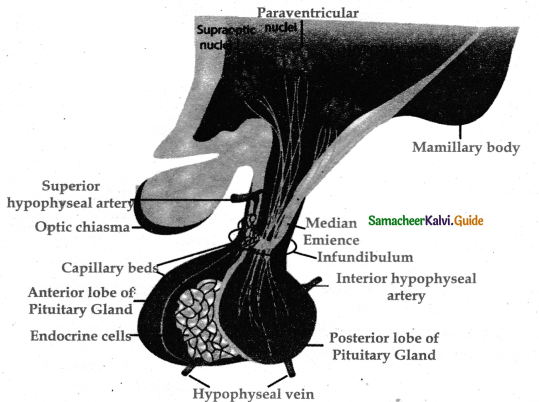
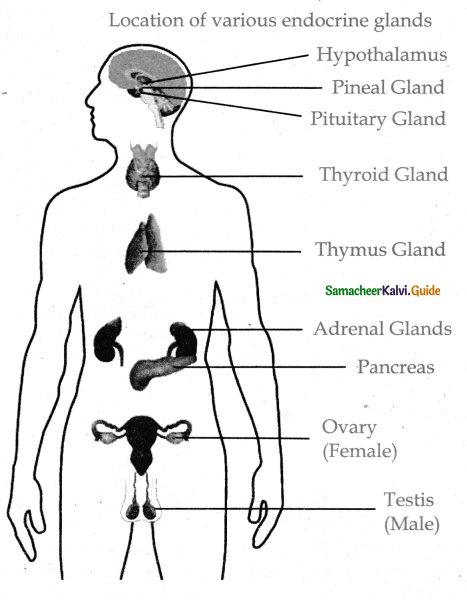
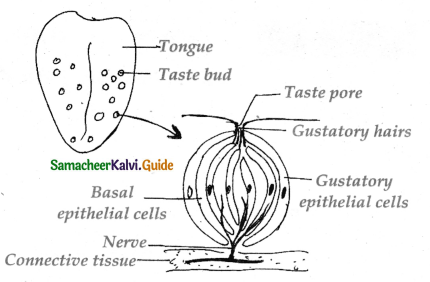
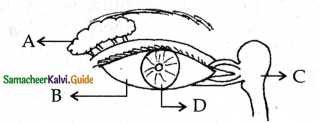
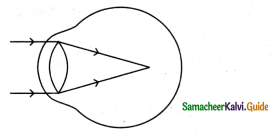
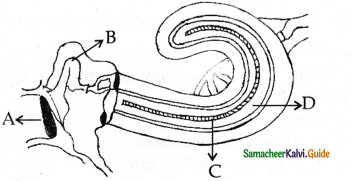
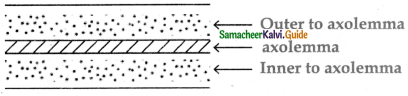
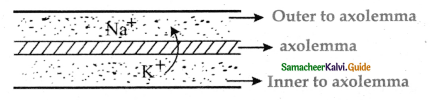
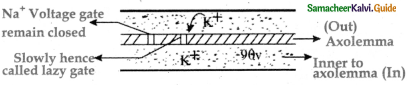
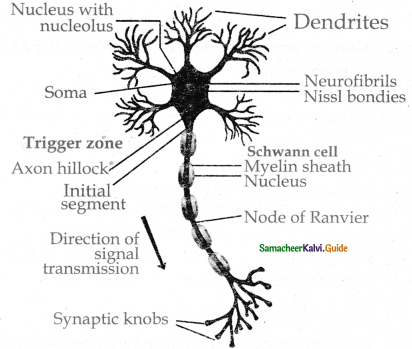
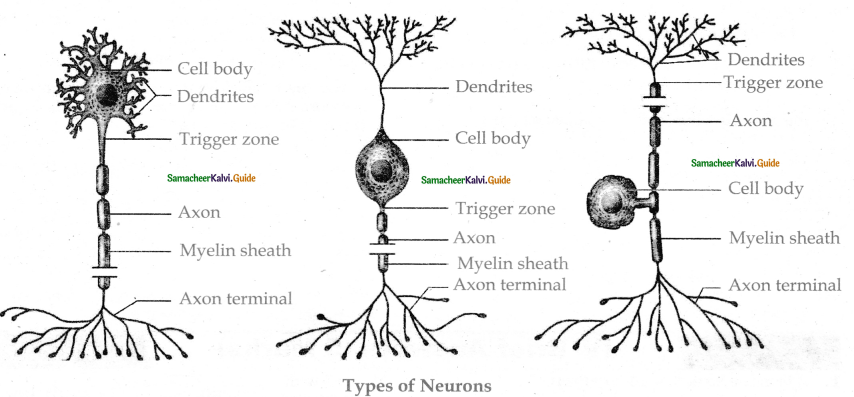
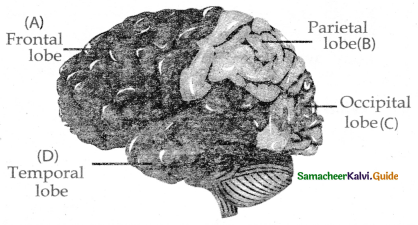
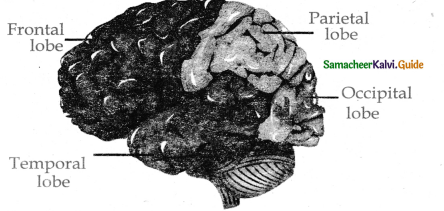
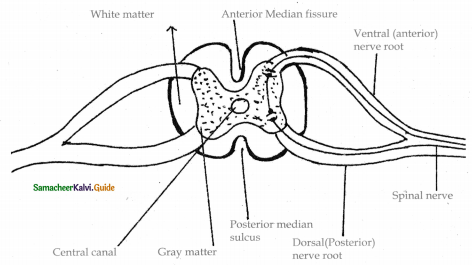
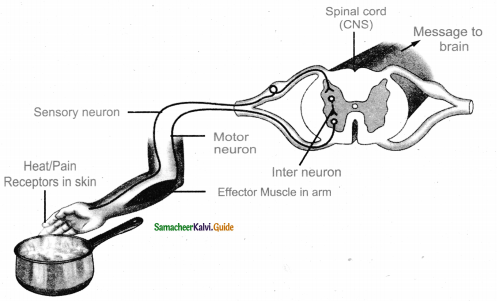
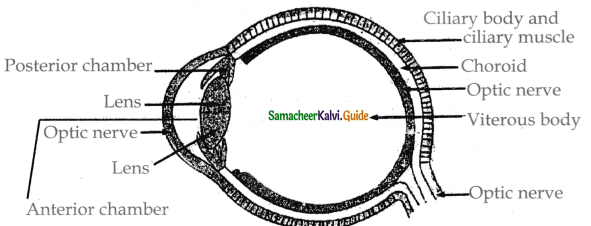
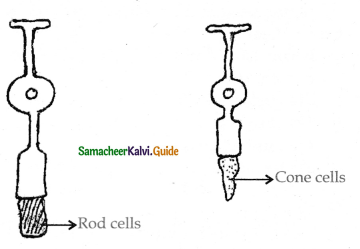
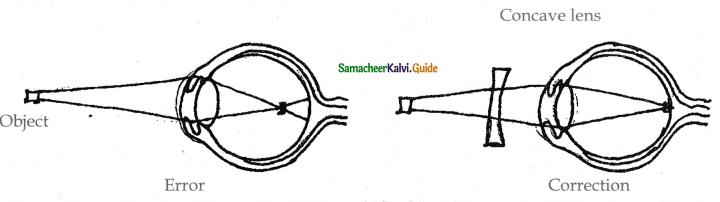
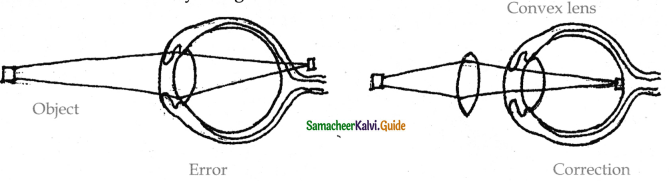
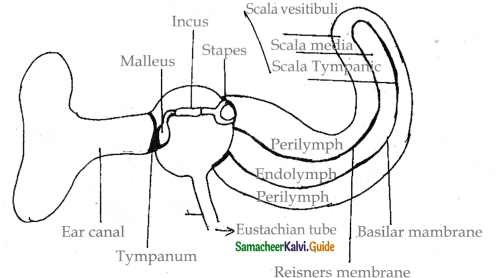
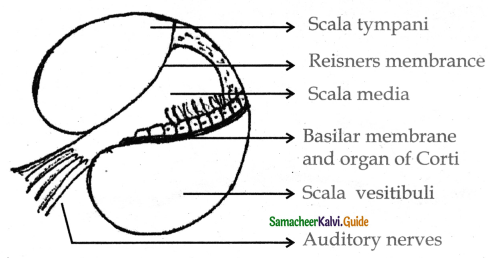
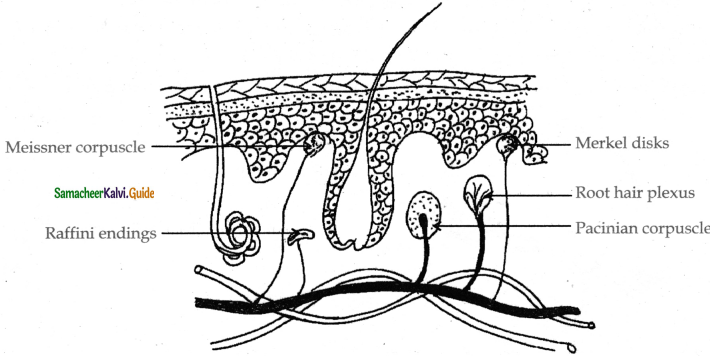
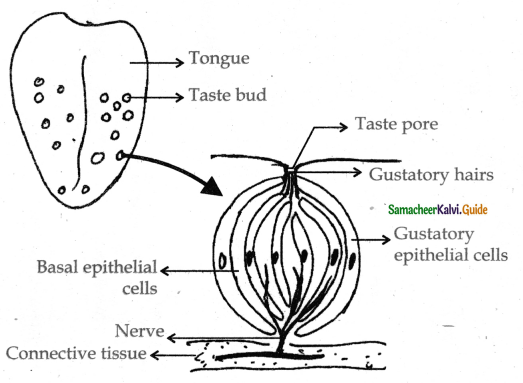
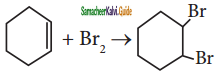
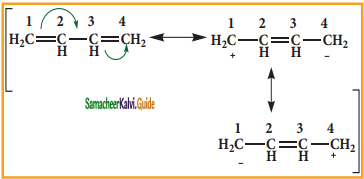
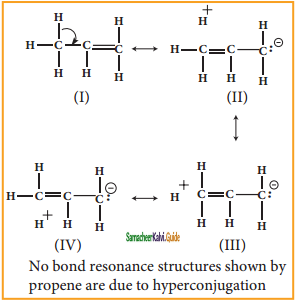
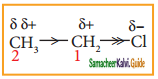
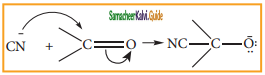
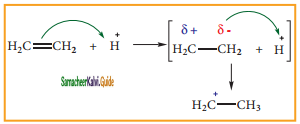
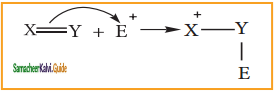
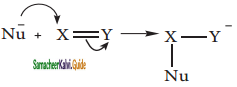
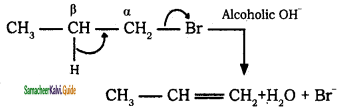
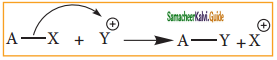
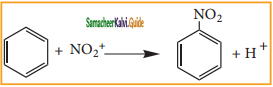
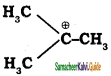 is
is