Tamilnadu State Board New Syllabus Samacheer Kalvi 11th Chemistry Guide Pdf Chapter 15 Environmental Chemistry Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 11th Chemistry Solutions Chapter 15 Environmental Chemistry
11th Chemistry Guide Environmental Chemistry Text Book Back Questions and Answers
Textbook Evaluation:
I. Choose the best answer:
Question 1.
The gaseous envelope around the earth is known as atmosphere. The region lying between an altitudes of 11 – 50 km is _______.
(a) Troposphere
(b) Mesosphere
(c) Thermosphere
(d) Stratosphere
Answer:
(d) Stratosphere
Question 2.
Which of the following belongs to secondary air pollutant?
(a) Hydrocarbon
(b) Peroxy acetyl nitrate
(c) Carbon monoxide
(d) Nitric oxide
Answer:
(a) Hydrocarbon
Question 3.
Which of the following is natural and human disturbance in ecology?
(a) Forest fire
(b) Floods
(c) Acid rain
(d) Green house effect
Answer:
(b) Floods
Question 4.
Bhopal Gas Tragedy is a case of _______.
(a) thermal pollution
(b) air pollution
(c) nuclear pollution
(d) land pollution
Answer:
(c) nuclear pollution
Question 5.
Haemoglobin of the blood forms carboxy haemoglobin with
(a) Carbon dioxide
(b) Carbon tetra chloride
(c) Carbon monoxide
(d) Carbonic acid
Answer:
(c) Carbon monoxide
![]()
Question 6.
Which sequence for green house gases is based on GWP?
(a) CFC > N2O > CO2 > CH4
(b) CFC > CO2 > N2O > CH4
(c) CFC > N2O > CH4 > CO2
(d) CFC > CH4 > N2O > CO2
Answer:
(b) CFC > CO2 > N2O > CH4
Question 7.
Photo chemical smog formed in congested metropolitan cities mainly consists of
(a) Ozone, SO2 and hydrocarbons
(b) Ozone, PAN and NO2
(c) PAN, smoke and SO2
(d) Hydrocarbons, SO2 and CO2
Answer:
(d) Hydrocarbons, SO2 and CO2
Question 8.
The pH of normal rain water is
(a) 6.5
(b) 7.5
(c) 5.6
(d) 4.6
Answer:
(c) 5.6
Question 9.
Ozone depletion will cause
(a) forest fires
(b) eutrophication
(c) bio magnification
(d) global warming
Answer:
(a) forest fires
Question 10.
_______ is considered to be ozone friendly substitude for CFC’S
(a) HFC (Hydro Fluro Carbon)
(b) Halons
(c) PAN (Peroxy acetyl nitrate)
(d) PAH (Poly cyclic aromatic hydro carbon)
Answer:
(d) PAH (Poly cyclic aromatic hydro carbon)
![]()
Question 11.
Identify the wrong statement in the following.
(a) The clean water would have a BOD value of less than 5 ppm
(b) Greenhouse effect is also called as Global warming
(c) Minute solid particles in air is known as particulate pollutants
(d) Biosphere is the protective blanket of gases surrounding the earth
Answer:
(c) Minute solid particles in air is known as particulate pollutants
Question 12.
Living in the atmosphere of CO is dangerous because it
(a) combines with O2 present inside to form CO2
(b) Reduces organic matter of tissues
(c) Combines with haemoglobin and makes it incapable to absorb oxygen
(d) Dries up the blood
Answer:
(c) Combines with haemoglobin and makes it incapable to absorb oxygen
Question 13.
World Ozone layer protection Day is celebrated in ________.
(a) June 5
(b) Nov – 19
(c) Sep – 16
(d) Jan – 26
Answer:
(c) Sep – 16
Question 14.
Release of oxides of nitrogen and hydrocarbons into the atmosphere by motor vehicles is prevented by using
(a) grit chamber
(b) scrubbers
(c) trickling filters
(d) catalytic convertors
Answer:
(b) scrubbers
Question 15.
Biochemical oxygen Demand value less than 5 ppm indicates a water sample to be
(a) highly polluted
(b) poor in dissolved oxygen
(c) rich in dissolved oxygen
(d) low COD
Answer:
(d) low COD
![]()
Question 16.
Match the List I with List II and select the correct answer using the code given below the lists:
| List I | List II |
| A. Depletion of ozone layer | 1. CO2 |
| B. Acid rain | 2. NO |
| C. Photochemical smog | 3. SO2 |
| D. Green house effect | 4. CFC |
Code:
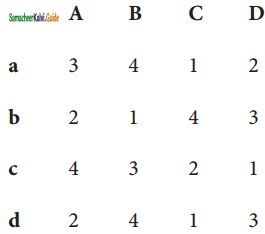
Answer:
(a)
Question 17.
Match the List I with List II and select the correct answer using the code given below the lists.
| List I | List II |
| A. Stone leprosy | 1. CO |
| B. Biological magnification | 2. Green house gases |
| C. Global warming | 3. Acid rain |
| D. Combination with haemoglobin | 4. DDT |
Code:

Answer:
(d)
Question 18.
Assertion (A):
If BOD level of water in a reservoir is more than 5 pm it is highly polluted.
Reason (R):
High biological oxygen demand means high activity of bacteria in water.
(i) Both (A) and (R) are correct and (R) is the correct explanation of (A)
(ii) Both (A) and (R) are correct and (R) is not the correct explanation of (A)
(iii) Both (A) and (R) are not correct
(iv) (A) is correct but (R) is not correct
(a) i
(b) ii
(c) iii
(d) iv
Answer:
(a) i
Question 19.
Assertion (A):
Excessive use of chlorinated pesticide causes soil and water pollution.
Reason (R):
Such pesticides are non – biodegradable.
(i) Both (A) and (R) are correct and (R) is the correct explanation of (A)
(ii) Both (A) and (R) are correct and (R) is not the correct explanation of (A)
(iii) Both (A) and (R) are not correct
(iv) (A) is correct but (R) is not correct
(a) i
(b) ii
(c) iii
(d) iv
Answer:
(a) i
Question 20.
Assertion (A):
Oxygen plays a key role in the troposphere
Reason (R):
Troposphere is not responsible for all biological activities
(i) Both (A) and (R) are correct and (R) is the correct explanation of (A)
(ii) Both (A) and (R) are correct and (R) is not the correct explanation of (A)
(iii) Both (A) and (R) are not correct
(iv) (A) is correct but (R) is not correct
(a) i
(b) ii
(c) iii
(d) iv
Answer:
(b) ii
![]()
II. Write brief answer to the following questions:
Question 21.
Dissolved oxygen in water is responsible for aquatic life. What processes are responsible for the reduction in dissolved oxygen in water?
Answer:
The process which are responsible for the reduction of dissolved oxygen in water are excessive use of phosphatic and nitrate fertilizers, detergents, the discharge of human sewage and organic waste from food, paper and pulp industries. The microorganisms which oxidize organic matter also used oxygen dissolved in H2O.
More over, during night, photosynthesis stops but the aquatic plants continue to respire, resulting in reduction of dissolved oxygen.
Question 22.
What would happen, if the greenhouse gases were totally missing in the earth’s atmosphere?
Answer:
The solar energy radiated back from the earth surface in absorbed by the green house gases. (CO2, CH4, O3, CFCs) are present near the earth’s surface. They heat up the atmosphere near the earth’s surface and keep it warm.
As a result of these, there is growth of vegetation which supports the life. In the absence of this effect, there will be not life of both plant and animal on the surface of the earth.
Question 23.
Define smog.
Answer:
Smog is a combination of smoke and fog which forms droplets that remain suspended in the air. Smog is a chemical mixture of gases that forms a brownish yellow haze over urban cities.Smog mainly consists of ground level ozone, oxides of nitrogen, volatile organic compounds, SO2 acidic aerosols and gases, and particulate matter.
Question 24.
Which is considered to be earth’s protective umbrella? Why?
Answer:
At high altitudes to the atmosphere consists of a layer of ozone (O3) which acts as an umbrella or shield for harmful UV radiations. It protects us from harmful effect such as skin cancer. UV radiation can convert molecular oxygen into ozone as shown in the following reaction.
O2(g) ![]() O(g) + O(g)
O(g) + O(g)
O(g) + O2(g) ![]() O3(g)
O3(g)
Question 25.
What are degradable and non – degradable pollutants?
Answer:
The pollutants are classified as bio-degradable and non-biodegradable pollutants.
Bio-degradable pollutants:
The pollutants which can be easily decomposed by the natural biological processes are called bio-degradable pollutants.
Example:
plant wastes, animal wastes etc.
Non bio-degradable pollutants:
The pollutants which cannot be decomposed by the natural biological processes are called Non bio-degradable
pollutants.
Examples:
Metal wastes (mainly Hg and Pb), D.D.T, plastics, nuclear wastes etc.,
These pollutants are harmful to living organisms even in low concentration. As they are not degraded naturally, it is difficult to eliminate them from our environment.
![]()
Question 26.
From where does ozone come in the photo chemical smog?
Answer:
NO2 ![]() NO + (O)
NO + (O)
O3 are strong oxidizing agent and can react with unburnt hydrocarbons in polluted air to form formaldehyde, acrolein and peroxy acetyl nitrate (PAN).
Question 27.
A person was using water supplied by corporation. Due to shortage of water he started using underground water. He felt laxative effect. What could be the cause?
Answer:
A moderate concentration of sulphate ions in water are harmless but excessive concentration is greater than 500 ppm in water causes laxative effects. Hence under ground water may have consisted excess of suplhates.
Question 28.
What is green chemistry?
Answer:
Efforts to control environmental pollution resulted in development of science for synthesis of chemicals favorable to environment which is called green chemistry. Green chemistry means science of environmentally favorable chemical synthesis.
Question 29.
Explain how does green house effect cause global warming.
Answer:
Greenhouse effect may be defined as the heating up of the earth surface due to trapping of infrared radiations reflected by earth’s surface by CO2 layer in the atmosphere”. The heating up of earth through the greenhouse effect is called global warming.
Without the heating caused by the greenhouse effect, Earth’s average surface temperature would be only about -18 °C (CPF). Although the greenhouse effect is a naturally occurring phenomenon, it is intensified by the continuous emission of greenhouse gases into the atmosphere.
During the past 100 years, the amount of carbon dioxide in the atmosphere increased by roughly 30 percent and the amount of methane more than doubled. If these trends continue, the average global temperature will increase which can lead to melting of polar ice caps and flooding of low lying areas. This will increase incidence of infectious diseases like dengue, malaria etc.
Question 30.
Mention the standards prescribed by BIS for quality of drinking water.
Answer:
Standard characteristics prescribed for deciding the quality of drinking water by BIS, in 1991 are shown in Table.
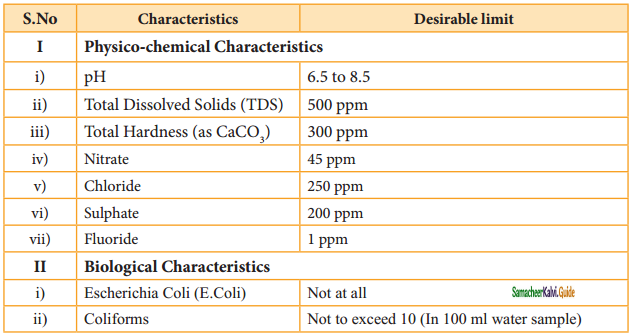
![]()
Question 31.
How does classical smog differ from photochemical smog?
Answer:
Classical smog was first observed in London in December 1952 and hence it is also known as Londo coal smoke and fog.
It occurs in cool atmospheric smog found in many large cities. The chemical composition is the mixture of SO2, SO3 and humidity. It generally occurs in the morning and becomes worse when the sun rises. This is mainly due to the induced oxidation of SO2 to SO3, which reacts with water yielding sulphuric acid aerosol.
Chemically it is reducing in nature because of high concentration of SO2 and so it is also called as reducing smog.
ii) Photo chemical smog or Los Angel Smog:
Photo Chemical smog was first observed in Los Angels in 1950. It occurs in warm, dry and sunny climate. This type of smog is formed by the combination of smoke, dust and fog with air pollutants like oxides of nitrogen and hydrocarbons in the presence of sunlight.
It forms when the sun shines and becomes worse in the afternoon. Chemically it is oxidizing in nature because of high concentration of oxidizing agents NO2 and O3, so it is also called as oxidizing smog.
Question 32.
What are particulate pollutants? Explain any three.
Answer:
1. Particulate pollutants are small solid particles and liquid droplets suspended in air. Many of particulate pollutants are hazardous.
Examples: dust, pollen, smoke, soot and liquid droplets (aerosols) etc,
2. Smoke particulate consists of solid particles (or) mixture of solid and liquid particles formed by the combustion of organic matter.
For example, cigarette smoke, oil smoke, smokes from burning of fossil fuel, garbage and dry leaves.
3. Dust composed of fine solid particles produced during crushing and grinding of solid materials.
For example, sand from sand blasting, saw dust from wood works, cement dust from cement factories and fly ash from power generating units.
Question 33.
Even though the use of pesticides increases crop production, they adversely affect the living organisms. Explain the function and the adverse effects of the pesticides.
Answer:
Pesticides are chemicals that are used to kill or stop the growth of unwanted organisms. But these pesticides can affect the health of human beings.
These are further classified as
Insecticides:
Insecticides like DDT, BHC, aldrin etc. can stay in soil for long period of time and are absorbed by soil. They contaminate root crops like carrot, raddish, etc.
Fungicide:
Organo mercury compounds are used as most common fungicide. They dissociate in soil to produce mercury which is highly toxic.
Herbicides:
Herbicides are the chemical compounds used to control unwanted plants. They are otherwise known as weed killers. Example sodium chlorate (NaClO3) and sodium arsenite (Na3AsO3). Most of the herbicides are toxic to mammals.
Question 34.
Ethane burns completely in air to give CO2, while in a limited supply of air gives CO. The same gases are found in automobile exhaust. Both CO and CO2 are atmospheric pollutants.
Answer:
The major pollutants of oxides of carbon are carbon monoxide and carbon dioxide.
(i) Carbon Monoxide:
Carbon monoxide is a poisonous gas produced as a result of incomplete combustion of coal are firewood. It is released into the air mainly by. automobile exhaust. It binds with haemoglobin and form carboxy haemoglobin which impairs normal oxygen transport by blood and hence the oxygen carrying capacity of blood is reduced. This oxygen deficiency results in headache, dizziness, tension, Loss of consciousness, blurring of eye sight and cardiac arrest.
(ii) Carbon dioxide:
Carbon dioxide is released into the atmosphere mainly by the process of respiration, burning of fossil fuels, forest fire, decomposition of limestone in cement industry etc.
Green plants can convert CO2 gas in the atmosphere into carbohydrate and oxygen through a process called photosynthesis. The increased CO2 level in the atmosphere is responsible for global warming. It causes headache and nausea.
Question 35.
On the basis of chemical reactions involved, explain how do CFC’s cause depletion of ozone layer in stratosphere?
Answer:
In the presence of uv radiation, CFC’s break up into chlorine free radical
CF2Cl2 ![]() CF2Cl + Cl
CF2Cl + Cl
CFCl3 ![]() CFCl2 + Cl
CFCl2 + Cl
Cl + O3 → ClO + O2
ClO + O → Cl + O2
Chlorine radical is regenerated in the course of reaction. Due to this continuous attack of Cl thinning of Ozone layer takes place which leads to formation of the ozone hole.
It is estimated that for every reactive chlorine atom generated in the stratosphere 1,00,000 molecules of ozone are depleted.
![]()
Question 36.
How is acid rain formed? Explain its effect.
Answer:
Rain water normally has a pH of 5.6 due to dissolution of atmospheric CO2 into it. Oxides, of sulphur and nitrogen in the atmosphere may be absorbed by droplets of water that make up clouds and get chemically converted into sulphuric acid and nitric acid respectively as a results of pH of rainwater drops to the level 5.6 hence it is called acid rain.
Acid rain is a by-product of a variety of sulphur and nitrogen oxides in the atmosphere. Burning of fossil fuels (coal and oil) in power stations, furnaces and petrol, diesel in motor engines produce sulphur dioxide and nitrogen oxides. The main contributors to acid rain are SO2 and NO2. They are converted into sulphuric acid and nitric acid respectively by the reaction with oxygen and water.
2SO2 + O2 + 2H2O → 2H2SO4
4NO2 + O2 + 2H2O → 4HNO3
Question 37.
What is sewage? What are the major steps involved in the treatment of sewage waste?
Answer:
Objectives of waste water treatment:
- To convert harmful compounds into harmless compounds.
- To eliminate the offensive smell.
- To remove the solid content of the sewage.
- To destroy the disease-producing micro organisms.
Treatment process:
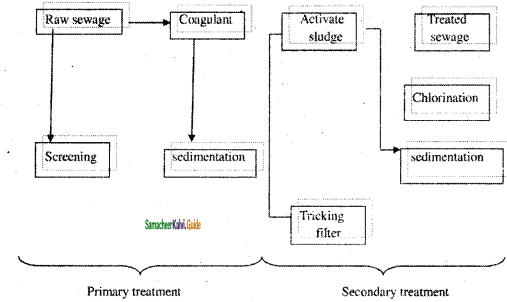
The sewage (or) wastewater treatment process involves the following steps.
I. Preliminary Treatment:
In this treatment, coarse solids and suspended impurities are removed by passing the wastewater through bar and mesh screens.
II. Primary treatment (or) Settling process:
In this treatment, greater proportion of the suspended inorganic and organic solids are removed from the liquid sewage by settling. In order to facilitate quick settling coagulants like alum, ferrous sulphate are added. These produce large gelatinous precipitates, which entrap finely divided organic matter and settle rapidly.
Al2(SO4)3 + 6H2O → 2Al(OH)3 ↓ + 3H2SO4
III. Secondary (or) biological treatment:
In this treatment, biodegradable organic impurities are removed by aerobic bacteria. It removes upto 90 % of the oxygen demanding wastes. This is done by trickling filter or activated sludge process.
(a) Trickling filter process:
It is a circular tank and is filled with either coarse or crushed rock. Sewage is sprayed over this bed by means of slowly rotating arms.
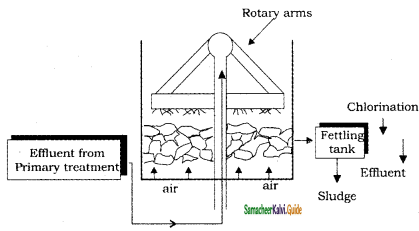
When sewage starts percolating downwards, mocroorganisms present in the sewage grow on the surface of filtering media using organic material of the sewage as food. After completion of aerobic oxidation the treated sewage is taken to the settling tank and the sludge is removed. This process removes about 80 – 85 % of BOD.
(b) Activated sludge process:
Activated sludge is biologically active sewage and it has a large number of aerobic bacterias, which can easily oxidize the organic impurities.
The sewage effluent from primary treatment is mixed with the required amount of activated sludge. Then the mixture is aerated in the aeration tank. Under these condition, organic impurities of the sewage get oxidized rapidly by the microorganisms.
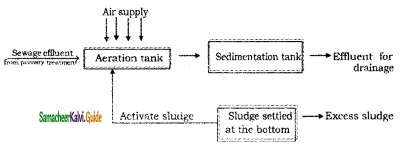
After aeration, the sewage is taken to the sedimentation tank. Sludges settle down in this tank, called activated sludge, a portion of which is used for seeding fresh batch of the sewage. This process removes about 90-95 % of BOD.
IV. Tertiary treatment:
After the secondary treatment, the sewage effluent has a lower BOD (25 ppm), which can be removed by the tertiary treatment process.
In the tertiary treatment, the effluent is introduced into a flocculation tank, where lime is added to remove phosphates. From the flocculation tank the effluent is led to ammonia stripping tower, where pH is maintained to 11 and the NH4+ is converted to gaseous NH3. Then the effluent is allowed to pass through activated charcoal column, where minute organic wastes are absorbed by charcoal. Finally the effluent water is treated with disinfectant (chlorine).
V. Disposal of sludge:
This is the last stage in the sewage treatment. Sludge formed from different steps can be disposed by
- dumping into low – lying areas,
- burning of sludge (incineration),
- dumping into the sea,
- using it as low grade fertilizers.
![]()
Question 38.
Differentiate the following:
(i) BOD and COD
(ii) Viable and non-viable particulate pollutants.
Answer:
(i) BOD and COD Biochemical oxygen demand (BOD):
The total amount of oxygen in milligrams consumed by microorganisms in decomposing the waste in one litre of water at 200°C for a period of 5 days is called biochemical oxygen demand (BOD) and its value is expressed in ppm. BOD is used as a measure of degree of water pollution. Clean water would have BOD value less than 5 ppm whereas highly polluted water has BOD value of 17 ppm or more.
Chemical Oxygen Demand (COD):
BOD measurement takes 5 days so another parameter called the Chemical Oxygen Demand (COD) is measured. Chemical oxygen demand (COD) is defined as the amount of oxygen required by the organic matter in a sample of water for its oxidation by a strong oxidising agent like K2Cr2O7 in acid medium for a period of 2 hrs.
(ii) Viable and non – viable particulate pollutants:
Viable particulates:
The viable particulates are the small size living organisms such as bacteria, fungi, moulds, algae, etc. which are dispersed in air. Some of the fungi cause allergy in human beings and diseases in plants.
Non-viable particulates:
The non- viable particulates are small solid particles and liquid droplets suspended in air. They help in the transportation of viable particles. There are four types of non-viable particulates in the atmosphere.
Example:
Smoke, Dust, Mists, Fumes.
Question 39.
Explain how oxygen deficiency is cause by carbon monoxide in our blood? Give its effect.
Answer:
Carbon monoxide is a poisonous gas produced as a result of incomplete combustion of coal are firewood. It is released into the air mainly by automobile exhaust. It binds with haemoglobin and form carboxy haemoglobin which impairs normal oxygen transport by blood and hence the oxygen carrying capacity of blood is reduced.
This oxygen deficiency results in headache, dizziness, tension, Loss of consciousness, blurring of eye sight and cardiac arrest. Efforts to control environmental pollution have resulted in development of science for synthesis of chemical favorable to environment and it is called green chemistry.
Question 40.
What are the various methods you suggest to protect our environment from pollution?
Answer:
- Waste management:
Environmental pollution can be controlled by proper disposal of wastes. - Recycling:
A large amount of disposed waste material can be reused by recycling the waste, thus it reduces the land fill and converts waste into useful forms. - Substitution of less toxic solvents for highly toxic ones used in certain industrial processes.
- Use of fuels with lower sulphur content (e.g., washed coal)
- Growing more trees.
- Control measures in vehicle emissions are adequate.
- Efforts to control environmental pollution have resulted in development of science for synthesis of chemical favourable to environment and it is called green chemistry.
![]()
11th Chemistry Guide Environmental Chemistry Additional Questions and Answers
I. Choose the best answer:
Question 1.
The type of pollution cause by spraying of DDT is
(a) air and soil
(b) air and water
(c) air
(d) air, water and soil
Answer:
(d) air, water and soil
Question 2.
The green house effect is caused by
(a) CO2
(b) NO2
(c) NO
(d) CO
Answer:
(a) CO2
Question 3.
The gas responsible for ozone depletion:
(a) NO and freons
(b) SO2
(c) CO2
(d) CO
Answer:
(a) NO and freons
Question 4.
In Antartica ozone depletion is due to the formation of following compound
(a) acrolein
(b) peroxyacetyl nitrate
(c) SO2 and NO2
(d) chlorine nitrate
Answer:
(a) acrolein
Question 5.
The main element of smog is
(a) O3 and PAN
(b) O3
(c) PAN
(d) PPN and PBN
Answer:
(a) O3 and PAN
![]()
Question 6.
Classical smog occurs in places of
(a) excess SO2
(b) low temperature
(c) high temperature
(d) excess NH3
Answer:
(b) low temperature
Question 7.
Which gas is responsible for ‘Bhopal Gas Tragedy’ in 1984?
(a) CO
(b) Methyl isocynate
(c) SO2 and NO2
(d) Ethyl isocynate
Answer:
(b) Methyl isocynate
Question 8.
Which gas is a main reason behind air pollution, is produced by
(a) sewage pollutant
(b) aerosols
(c) industrial remains
(d) Above all
Answer:
(b) aerosols
Question 9.
Which is a dangerous radiological pollutant?
(a) C14
(b) S35
(c) Sr90
(d) P32
Answer:
(c) Sr90
Question 10.
Which is related to ‘Green House Effect’?
(a) Farming of Green Plants
(b) Farming of Vegetables in Houses
(c) Global Warming
(d) Biodegradable pollutant
Answer:
(c) Global Warming
![]()
Question 11.
The uppermost region of the atmosphere is called
(a) Ionosphere
(b) Mesosphere
(c) Troposphere
(d) Stratosphere
Answer:
(d) Stratosphere
Question 12.
Which of the following is the coldest region of atmosphere
(a) Thermosphere
(b) Mesosphere
(c) Troposphere
(d) Stratosphere
Answer:
(b) Mesosphere
Question 13.
The region which is greatly affected by air pollution is
(a) Thermosphere
(b) Stratosphere
(c) Troposphere
(d) Mesosphere
Answer:
(c) Troposphere
Question 14.
The substance which is a primary pollutant?
(a) H2SO4
(b) CO
(c) PAN
(d) Aldehydes
Answer:
(b) CO
Question 15.
Depletion of ozone layer causes
(a) breast cancer
(b) blood cancer
(c) lung cancer
(d) skin cancer
Answer:
(d) skin cancer
![]()
Question 16.
Formation of London smog takes place in
(a) Winter during day time
(b) summer during day time
(c) summer during morning time
(d) winter during morning time
Answer:
(d) winter during morning time
Question 17.
The substance which is not regarded as a pollutant?
(a) NO2
(b) CO2
(c) O3
(d) Hydrocarbons
Answer:
(b) CO2
Question 18.
Green house gases
(a) allow shorter wavelength to enter earth’s atmosphere while doesn’t allow longer wavelength to leave the earth’s atmosphere.
(b) allow longer wavelength to enter earth atmosphere while doesn’t allow shorter wavelength to leave the surface.
(c) don’t have wavelength specific character.
(d) she wavelength specific behaviour near the earth while far from earth these have wavelength independent behavior.
Answer:
(a) allow shorter wavelength to enter earth’s atmosphere while doesn’t allow longer wavelength to leave the earth’s atmosphere.
Question 19.
Carbon monoxide (CO) is harmful to man because
(a) it forms carbolic acid
(b) it generates excess CO2
(c) it is carcinogenic
(d) it competes with O2 for haemoglobin
Answer:
(d) it competes with O2 for haemoglobin
Question 20.
Today the concentration of green house gases is very high because of
(a) use of refrigerator
(b) increased combustion of oils and coal
(c) deforestation
(d) All of the above
Answer:
(d) All of the above
![]()
Question 21.
The quantity of CO2 in atmosphere is
(a) 3.34 %
(b) 6.5 %
(c) 0.034 %
(d) 0.34 %
Answer:
(c) 0.034 %
Question 22.
BOD of pond is connected with
(a) microbes & organic matter
(b) organic matter
(c) microbes
(d) None of these
Answer:
(a) microbes & organic matter
Question 23.
When rain is accompanied by a thunderstorm, the collected rain water will have a pH value
(a) slightly lower than that of rain water without thunderstorm
(b) slightly higher than that when the thunderstorm is not there
(c) uninfluenced by occurrence of thunderstorm
(d) which depends upon the amount of dust in air
Answer:
(a) slightly lower than that of rain water without thunderstorm
Question 24.
Water pollution is caused by
(a) pesticides
(b) SO2
(c) O2
(d) CO2
Answer:
(a) pesticides
Question 25.
Minamata disease of Japan is due to pollution of
(a) Aresenic
(b) Lead
(c) Cynide
(d) Mercury
Answer:
(d) Mercury
![]()
Question 26.
Which causes death of fish in water bodies polluted by sewage?
(a) Foul smell
(b) Pathogens
(c) Herbicides
(d) Decrease in D.O.
Answer:
(d) Decrease in D.O.
Question 27.
Sewage water is purified by
(a) aquatic plants
(b) microorganisms
(c) light
(d) fishes
Answer:
(b) microorganisms
Question 28.
Which pollutant is harmful for ‘Tajmahal’?
(a) Hydrogen
(b) O2
(c) SO2
(d) Chlorine
Answer:
(c) SO2
Question 29.
Negative soil pollution is
(a) reduction in soil productivity due to erosion and over use
(b) reduction in soil productivity due to addition of pesticides and industrial wastes
(c) converting fertile land into barren land by dumping ash, sludge and garbage
(d) None of the above
Answer:
(a) reduction in soil productivity due to erosion and over use
Question 30.
The quantity of DDT in food chain
(a) decreases
(b) remains same
(c) increases
(d) changes
Answer:
(c) increases
![]()
Question 31.
Which is known as “Third poison of environment” and also creates ‘Blue baby syndrome’
(a) Nitrate present in water
(b) Phosphate and detergents found in water
(c) Cyanide
(d) Pesticides
Answer:
(b) Phosphate and detergents found in water
Question 32.
The substance having the largest concentration in acid rain?
(a) H2CO3
(b) HNO3
(c) HCl
(d) H2SO4
Answer:
(d) H2SO4
Question 33.
Water is often treated with chlorine to
(a) remove hardness
(b) increase oxygen content
(c) kill germs
(d) remove suspended particles
Answer:
(c) kill germs
Question 34.
Thermal pollution affects mainly
(a) vegetation
(b) aquatic creature
(c) rocks
(d) air
Answer:
(b) aquatic creature
Question 35.
B.O.D test or biochemical oxygen demand test is made for measuring
(a) air pollution
(b) water pollution
(c) noise pollution
(d) soil pollution
Answer:
(b) water pollution
![]()
Question 36.
Brewery and sugar factory water alters the quality of a water body by increasing
(a) temperature
(b) turbidity
(C) pH
(d) COD and BOD
Answer:
(d) COD and BOD
Question 37.
A dental disease characterized by mottling of teeth is due to the presence of a certain chemical element in drinking water. Which is that element?
(a) Boron
(b) Chlorine
(c) Fluorine
(d) Mercury
Answer:
(c) Fluorine
Question 38.
The high amount of E.coli in water is an indicator of
(a) hardness of water
(b) industrial pollution
(c) sewage pollution
(d) presence of chlorine in the water
Answer:
(c) sewage pollution
Question 39.
A lake with an inflow of domestic sewage rich in organic waste may result in
(a) drying of the lake very soon due to algal bloom
(b) an increase production of fish due to a lot of nutrients
(c) death of fish due to lack of oxygen
(d) increased population of aquatic food web organisms
Answer:
(c) death of fish due to lack of oxygen
Question 40.
In which one of the following the BOD (Biochemical Oxygen Demand) of sewage(S), distillery effluent (DE), paper mill effluent (PE) and sugar mill effluent (SE) have been arranged in ascending order
(a) SE < S < PE < DE
(b) SE < PE < S < DE
(c) PE < S < SE < DE
(d) S < DE < PE < SE
Answer:
(c) PE < S < SE < DE
![]()
Question 41.
The greenhouse effect is because of the
(a) presence of gases, which in general are strong infrared absorbers, in the atmosphere
(b) presence of CO2 only in the atmosphere
(c) presence of O3 and CH4 in the atmosphere
(d) N2O and chlorofluoro hydrocarbons in the atmosphere
Answer:
(a) presence of gases, which in general are strong infrared absorbers, in the atmosphere
Question 42.
Which of the following is/are the hazardous pollutant(s) present in automobile exhaust gases?
(a) N2
(b) CO
(c) CH4
(d) Oxides of nitrogen
Answer:
(c) CH4
Question 43.
Green chemistry means such reactions which:
(a) produce colour during reactions
(b) reduce the use and production of hazardous chemicals
(c) are related to the depletion of ozone layer
(d) study the reactions in plants
Answer:
(b) reduce the use and production of hazardous chemicals
Question 44.
Which one of the following statement is not true?
(a) pH of drinking water should be between 5.5 – 9.5.
(b) Concentration of DO below 6 ppm is good for the growth of fish.
(c) Clean water would have a BOD value of less than 5 ppm.
(d) Oxides of sulphur, nitrogen and carbon are the most widespread air pollutant.
Answer:
(b) Concentration of DO below 6 ppm is good for the growth of fish.
Question 45.
Which one of the following statements regarding photochemical smog is not correct?
(a) Carbon monoxide does not play any role in photochemical smog formation.
(b) photochemical smog is an oxidizing agent in character.
(c) photochemical smog is formed through photochemical reaction involving solar energy.
(d) Photochemical smog does not cause irritation in eyes and throat.
Answer:
(d) Photochemical smog does not cause irritation in eyes and throat.
![]()
Question 46.
Frequent occurrence of water blooms in a lake indicates
(a) nutrient deficiency
(b) oxygen deficiency
(c) excessive nutrient availability
(d) absence of herbivores in the lake
Answer:
(b) oxygen deficiency
Question 47.
The smog is essentially caused by the presence of
(a) Oxides of sulphur and nitrogen
(b) O2 and N2
(c) O2 and O3
(d) O2 and N2
Answer:
(a) Oxides of sulphur and nitrogen
Question 48.
Identify the wrong statement in the following.
(a) Chlorofluorocarbons are responsible for ozone layer depletion.
(b) Greenhouse effect is responsible for global warming.
(c) Ozone layer does not permit infrared radiation from the sun to reach the earth.
(d) Acid rain is mostly because of oxides of nitrogen and sulphur.
Answer:
(c) Ozone layer does not permit infrared radiation from the sun to reach the earth.
Question 49.
Identify the incorrect statement from the following.
(a) Ozone absorbs the intense ultraviolet radiation of the sun.
(b) Depletion of ozone layer is because of its chemical reactions with chlorofluoro alkanes.
(c) Ozone absorbs infrared radiation.
(d) Oxides of nitrogen in the atmosphere can cause the depletion of ozone layer.
Answer:
(c) Ozone absorbs infrared radiation
Question 50.
What is DDT among the following?
(a) Greenhouse gas
(b) A fertilizer
(c) Biodegradable pollutant
(d) Non – biodegradable pollutant
Answer:
(d) Non – biodegradable pollutant
![]()
Question 51.
The gas leaked from a storage tank of the Union Carbide plant in Bhopal gas tragedy was:
(a) Methyl isocyanate
(b) Methylamine
(c) Ammonia
(d) Phosgene
Answer:
(a) Methyl isocyanate
Question 52.
Black – foot disease is caused due to groundwater contaminated with excess of
(a) Nitrate
(b) Fluoride
(c) Arsenic
(d) Sulphur
Answer:
(c) Arsenic
Question 53.
Exposure of an organism to UV system causes
(a) photodynamic action
(b) formation of thymidine
(c) splitting of H – bonds of DNA
(d) splitting of phosphodiester bonds
Answer:
(c) splitting of H – bonds of DNA
Question 54.
Under column – I, a list of gases that are known to have a greenhouse effect is given. Relate them to their main source selecting from the given under Column – II:
| Column – I | Column – II |
| A. Nitrous oxide | 1. Secondary pollutant from car exhausts |
| B. Chlorofluoro carbon (CFCs) | 2. Combustion of fossil fuels, wood, etc. |
| C. Methane | 3. Denitrification |
| D. Ozone (O3) | 4. refrigerators, aerosol, sprays |
| E. Carbondioxide | 5. Cattle, rice fields, toilets |
(a) A – 3, B – 4, C – 5, D – 1, E – 2
(b) A – 5, B – 1, C – 3, D – 4, E – 2
(c) A – 4, B – 5 , C – 1, D – 2, E – 3
(d) A – 1, B – 3, C – 4, D – 5, E – 2
Answer:
(a) A – 3, B – 4, C – 5, D – 1, E – 2
Question 55.
Minamata disease is a pollution related disease results form
(a) oil spills into sea
(b) accumulation of arsenic into atmosphere
(c) release of industrial waste mercury into bodies water
(d) release human organic waste into drinking water
Answer:
(c) release of industrial waste mercury into bodies water
![]()
Question 56.
Air pollution causing photochemical oxidants production include
(a) Carbon monoxide, sulphur dioxide
(b) Nitrous oxide, nitric acid fumes, nitric oxide
(c) Ozone, peroxyacetyl nitrate, aldehydes
(d) Oxygen, chlorine, fuming nitric acid
Answer:
(c) Ozone, peroxyacetyl nitrate, aldehydes
Question 57.
Photochemical smog formed in congested metropolitan cities mainly consists of
(a) ozone, peroxyacetyl nitrate and NOx
(b) smoke, peroxyacetyl nitrate and SO2
(c) hydrocarbons, SO2 and CO2
(d) hydrocarbons, ozone and SOx
Answer:
(a) ozone, peroxyacetyl nitrate and NOx
Question 58.
Which, one of the following statements is correct?
(a) Extensive use of chemical fertilizers may lead to eutrophication of nearby water bodies
(b) Both Azotobacter and Rhizobium fix atmospheric nitrogen in root nodules of plants
(c) Cyanobacteria such as Anabaena and Nostoc are important mobilizers of phosphates and potassium for plant nutrition in soil
(d) At present it is not possible to grow maize without chemical fertilizers
Answer:
(a) Extensive use of chemical fertilizers may lead to eutrophication of nearby water bodies
Question 59.
Reducing the use of non-biodegradable things will contribute of
(a) Increase in O2
(b) Cyanophycean blooms occur
(c) Depletion of O2 layers
(d) Eutrophication
Answer:
(a) Increase in O2
Question 60.
Which of the following metal is a water pollutant and causes sterility in human. being?
(a) As
(b) Mn
(c) Mg
(d) Hg
Answer:
(b) Mn
![]()
Question 61.
Lichens do not like to grow in cities
(a) because of absence of the right type of algae and fungi
(b) because of lack of moisture
(c) because of SO2 pollution
(d) because natural habitat is missing
Answer:
(c) because of SO2 pollution
Question 62.
Limit of BOD prescribed by Central pollution Control Board for the discharge of industrial and municipal waste waters into natural surface waters, is
(a) < 100 ppm
(b) < 30 ppm
(c) < 3.0 ppm
(d) < 10 ppm
Answer:
(b) < 30 ppm
Question 63.
Which one of the following pairs is mismatched
(a) Fossil fuel burning – release of CO2
(b) Nuclear power – radioactive wastes
(c) Solar energy – Greenhouse effect
(d) Biomass burning – release of CO2
Answer:
(c) Solar energy – Greenhouse effect
Question 64.
In a coal fired power plant electrostatic precipitators are installed to control emission of
(a) SO2
(b) NOx
(c) SPM
(d) CO
Answer:
(c) SPM
Question 65.
The term “Bio – magnification” refers to the
(a) growth of organism due to food consumption
(b) increase in population size
(c) blowing up of environmental issues by man
(d) increase in the concentration of non – degradable pollutants as they pass through food chain
Answer:
(d) increase in the concentration of non – degradable pollutants as they pass through food chain
![]()
Question 66.
In almost all Indian metropolitan cities like Delhi, the major atmospheric pollutant(s) is / are
(a) suspended particulate matter (SPM)
(b) oxides of sulphur
(c) carbon dioxide and carbon monoxide
(d) oxides of nitrogen
Answer:
(a) suspended particulate matter (SPM)
Question 67.
In coming years, skin related disorders will be more common due to
(a) pollutants in air
(b) use of detergents
(c) water pollution
(d) depletion of ozone layer
Answer:
(d) depletion of ozone layer
Question 68.
Statement 1:
Inhabitants close to very busy airports are likely to experience health hazards.
Statement 2:
Sound level of jet aeroplanes usually exceeds 160 dB.
(a) Statement – 1 is True, Statement – 2 is True, Statement – 2 is a correct explanation for Statement – 1.
(b) Statement – 1 is True, Statement – 2 is True, Statement – 2 is not a correct explanation for statement – 1
(c) Statement – 1 is True, Statement – 2 is False
(d) Statement – 1 is False, Statement – 2 is True
Answer:
(a) Statement – 1 is True, Statement – 2 is True, Statement – 2 is a correct explanation for Statement – 1.
Question 69.
Statement 1:
Suspended particulate matter (SPM) is an important pollutant released by diesel vehicles.
Statement 2:
Catalytic converters greatly reduce pollution caused by automobiles.
(a) Statement – 1 is True, Statement – 2 is True, Statement -2 is a correct explanation for Statement – 1.
(b) Statement – 1 is True, Statement – 2 is True, Statement – 2 is not a correct explanation for statement – 1
(c) Statement – 1 is True, Statement – 2 is False
(d) Statement – 1 is False, Statement – 2 is True
Answer:
(b) Statement – 1 is True, Statement – 2 is True, Statement – 2 is not a correct explanation for statement – 1
Question 70.
Statement 1:
Eutrophication shows increase in productivity in water.
Statement 2:
With increasing eutrophication, the diversity of the phytoplankton increases.
(a) Statement – 1 is True, Statement – 2 is True, Statement -2 is a correct explanation for Statement – 1.
(b) Statement – 1 is True, Statement – 2 is True, Statement – 2 is not a correct explanation for statement – 1
(c) Statement – 1 is True, Statement – 2 is False
(d) Statement – 1 is False, Statement – 2 is True
Answer:
(b) Statement – 1 is True, Statement – 2 is True, Statement – 2 is not a correct explanation for statement – 1
![]()
Question 71.
Statement 1:
The main cause of the Bhopal gas tragedy was phosgene.
Statement 2:
Phosgene is a volatile liquid.
(a) Statement – 1 is True, Statement – 2 is True, Statement -2 is a correct explanation for Statement – 1.
(b) Statement – 1 is True, Statement – 2 is True, Statement – 2 is not a correct explanation for statement – 1
(c) Statement – 1 is True, Statement – 2 is False
(d) Statement – 1 is False, Statement – 2 is True
Answer:
(d) Statement – 1 is False, Statement – 2 is True
Question 72.
Statement 1:
CO2 causes green house effect.
Statement 2:
Other gases do not show such effect.
(a) Statement – 1 is True, Statement – 2 is True, Statement -2 is a correct explanation for Statement – 1
(b) Statement – 1 is True, Statement – 2 is True, Statement – 2 is not a correct explanation for statement – 1
(c) Statement – 1 is True, Statement – 2 is False
(d) Statement – 1 is False, Statement – 2 is True
Answer:
(c) Statement – 1 is True, Statement – 2 is False
![]()
II. Very short question and answers (2 Marks):
Question 1.
What is called as environmental pollution?
Answer:
Any undesirable change in our environment that has harmful effects on plants, animals and human beings is called environmental pollution.
Question 2.
What are pollutants?
Answer:
The substances which cause pollution of environment are called pollutants.
Question 3.
Write the different types of atmospheric pollution.
Answer:
- Air pollution
- Water pollution
- Soil pollution.
Question 4.
What is Air pollution?
Answer:
Any undesirable change in air which adversely affects living organisms is called air pollution. Air pollution is limited to troposphere and stratosphere. Air pollution is mainly due to the excessive discharge of undesirable foreign matter in to the atmospheric air.
![]()
Question 5.
What are the techniques adopt to reduce particulate pollutants?
Answer:
The particulates from air can be removed by using electrostatic precipitators, gravity settling chambers, and wet scrubbers or by cyclone collectors. These techniques are based on washing away or settling of the particulates.
Question 6.
Define soil pollution.
Answer:
Soil pollution is defined as the buildup of persistent toxic compounds, radioactive materials, chemical salts and disease causing agents in soils which have harmful effects on plant growth and animal health.
Question 7.
Write the effects that were caused by classical smog.
Answer:
- Smog is primarily responsible for acid rain.
- Smog results in poor visibility and it affects air and road transport.
- It also causes bronchial irritation.
![]()
III. Short question and answers (3 Marks):
Question 1.
How the oxides of nitrogen pollute the atmospheric air?
Answer:
Oxides of nitrogen are produced during high temperature combustion processes, oxidation of nitrogen in air and from the combustion of fuels (coal, diesel, petrol etc.).
N2 + O2 ![]() 2NO
2NO
2NO + O2 ![]() 2NO2
2NO2
NO + O3 → NO2 + O2
The oxides of nitrogen are converted into nitric acid which comes, down in the form of acid rain. They also form reddish brown haze in heavy traffic. Nitrogen dioxide potentially damages plant leaves and retards photosynthesis. NO2 is a respiratory irritant and it can cause asthma and lung injury. Nitrogen dioxide is also harmful to various textile fibres and metals.
Question 2.
How the hydrocarbon compounds make harmful effects on living things?
Answer:
The compounds composed of carbon and hydrogen only are called hydrocarbons. They are mainly produced naturally (marsh gas) and also by incomplete combustion of automobile fuel.
They are potential cancer causing (carcinogenic) agents. For example, polynuclear aromatic hydrocarbons (PAH) are carcinogenic, they cause irritation in eyes and mucous membranes.
Question 3.
Explain the environmental impact of ozone depletion.
Answer:
The formation and destruction of ozone is a regular natural process, which never disturbs the equilibrium level of ozone in the stratosphere. Any change in the equilibrium level of the ozone in the atmosphere will adversely affect life in the biosphere in the following ways.
Depletion of ozone layer will allow more UV rays to reach the earth surface and layer would cause skin cancer and also decrease the immunity level in human beings. UV radiation affects plant proteins which leads to harmful mutation of cells. UV radiation affects the growth of phytoplankton, as a result ocean food chain is disturbed and even damages the fish productivity.
![]()
Question 4.
Write the causes of water pollution.
Answer:
(i) Microbiological (Pathogens):
Disease causing microorganisms like bacteria, viruses and protozoa are most serious water pollutants. They come from domestic sewage and animal excreta. Fish and shellfish can become contaminated and people who eat them can become ill. Some serious diseases like polio and cholera are water borne diseases. Human excreta contain bacteria such as Escherichia coli and Streptococcus faecalis which cause gastrointestinal diseases.
(ii) Organic wastes:
Organic matter such as leaves, grass, trash etc can also pollute water. Water pollution is caused by excessive phytoplankton growth within water. Microorganisms present in water decompose these organic matter and consume dissolved oxygen in water.
Question 5.
Write a short note on Eutrophication.
Answer:
Eutrophication is a process by which water bodies receive excess nutrients that stimulates excessive plant growth (algae, other plant weeds). This enhanced plant growth in water bodies is called as algae bloom.
The growth of algae in extreme abundance covers the water surface and reduces the oxygen concentration in water. Thus, bloom-infested water inhibits the growth of other living organisms in the water body. This process in which the nutrient-rich water bodies support a dense plant population, kills animal life by depriving it of oxygen and results in loss of biodiversity is known as eutrophication.
Question 6.
Write the harmful effects those caused by chemical water pollutants.
Answer:
- Cadmium and mercury can cause kidney damage.
- Lead poisoning can leads to severe damage of kidneys, liver, brain etc. it also affects central nervous system
- Polychlorinated biphenyls (PCBs) causes skin diseases and are carcinogenic in nature.
![]()
Question 7.
Distinguish between BOD and COD.
Answer:
| BOD | COD |
| 1. BOD is the amount of oxygen required for the biological decomposition of organic matter present in the water. | COD is the amount of oxygen required for chemical oxidation of organic matter using some oxidizing agent like K2Cr207 and KMn04. |
| 2. It is an important indication of the amount of organic matter present in the river water. | It is carried out to determine the pollution strength of river water. |
| 3. Since complete oxidation occurs in an indefinite period, the reaction period is taken as 5 days at 20°C. | It is a rapid process and takes only 8 hours. |
IV. Long question and answer (5 Marks):
Question 1.
Explain the different layers of earth’s atmosphere.
Answer:
Troposphere:
The lowest layer of the atmosphere is called the troposphere and it extends from o – 10 km from the earth surface. About 80% of the mass of the atmosphere is in this layer. This troposphere in further divided as follows.
i) Hydrosphere:
Hydrosphere includes all types of water sources like oceans, seas, rivers, lakes, streams, underground water, polar icecaps, clouds etc. It covers about 75% of the earth’s surface. Hence the earth is called as a blue planet.
ii) Lithosphere:
Lithosphere includes soil, rocks and mountains which are solid components of earth.
iii) Biosphere:
It includes the lithosphere, hydrosphere and atmosphere integrating the living organism present in the lithosphere, hydrosphere and atmosphere.
Question 2.
How the oxides of sulphur pollute the atmospheric air?
Answer:
Sulphur dioxide and sulphur trioxide are produced by burning sulphur-containing fossil fuels arid roasting sulphide ores. Sulphur dioxide is a poisonous gas to both animals and plants. Sulphur dioxide causes eye irritation, coughing and respiratory asthma, bronchitis, etc.
Sulphur dioxide is oxidised into more harmful sulphur trioxide in the presence of particulate matter present in polluted air.
2SO2 + O2 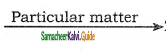 2SO3
2SO3
SO3 combines with atmospheric water vapour to form H2SO4, which comes down in the form of acid rain.
SO3 + H2O → H2SO4
Question 3.
Explain the health effects of particulate pollutants for human health.
Answer:
- Dust, mist, fumes,etc., are air borne particles which are dangerous for human health. Particulate pollutants bigger than 5 microns are likely to settle in the nasal passage whereas particles of about 10 micron enters the lungs easily and causes scaring or fibrosis of lung lining.
- They irritate the lungs and causes cancer and a,sthma. This disease is also called pneumoconiosis. Coal miners may suffer from black lung disease. Textile workers may suffer from white lung disease.
- Lead particulates affect children’s brain, interferes maturation of RBCs and even cause cancer.
- Particulates in the atmosphere reduce visibility by scattering and absorption of sunlight. It is dangerous for aircraft and motor vehicles
- Particulates provide nuclei for cloud formation and increase fog and rain.
- Particulates deposit on plant leaves and hinder the intake of CO2 from the air and affect photosynthesis.
![]()
Question 4.
Explain the effects of photo chemical smog and its control.
Answer:
The three main components of photo chemical smog are nitrogen oxide, ozone and oxidised hydro carbon like formaldehyde(HCHO), Acrolein (CH2 = CH – CHO), peroxy acetyl nitrate (PAN). Photochemical smog causes irritation to eyes, skin and lungs, increase in chances of asthma.
High concentrations of ozone and NO can cause nose and throat irritation, chest pain, uncomfortable in breathing, etc. PAN is toxic to plants, attacks younger leaves and cause bronzing and glazing of their surfaces. It causes corrosion of metals stones, building materials and painted surfaces.
Control of Photo chemical smog:
The formation of photochemical smog can be suppressed by preventing the release of nitrogen oxides and hydrocarbons into the atmosphere from the motor vehicles by using catalytic convertors in engines. Plantation of certain trees like Pinus, Pyrus, Querus Vitus and juniparus can metabolise nitrogen oxide.
Question 5.
List out the major water pollutants and their sources.
Answer:
| Pollutant | Sources |
| 1.Microorganisms | Domestic sewage, domestic waste water, dung heap. |
| 2. Organic wastes | Domestic sewage, animal excreta, food processing factory waste, detergents and decayed animals and plants. |
| 3. Plant nutrients | Chemical fertilisers. |
| 4. Heavy metals | Heavy metal producing factories. |
| 5. Sediments | Soil erosion by agriculture and strip-mining. |
| 6. Pesticides | Chemicals used for killing insects, fungi and weeds. |
| 7. Radioactive | Mining of uranium containing minerals substances. |
| 8. Heat | Water used for cooling in industries. |
Question 6.
What are the major sources that cause soil pollution?
Answer:
Artificial fertilizers:
Soil nutrients are useful for growth of plants. Plants obtains carbon, hydrogen and oxygen from air or water, whereas other essential nutrients like nitrogen, phosphorous, potassium, calcium, magnesium, sulphur are being absorbed from soil. To remove the deficiency of nutrients in soil, farmers add artificial fertilizers. Increased use of phosphate fertilizers or excess use of artificial fertilizers like NPK in soil, results in reduced yield in that soil.
Pesticides:
Pesticides are the chemicals that are used to kill or stop the growth of unwanted organisms. But these pesticides can affect the health of human beings.
These are further classified as
Insecticides:
Insecticides like DDT, BHC, aldrin etc. can stay in soil for long period of time and are absorbed by soil. They contaminate root crops like carrot, raddish, etc.
Fungicide:
Organo mercury compounds Eire used as most common fungicide. They dissociate in soil to produce mercury which is highly toxic.
Herbicides:
Herbicides are the chemical compounds used to control unwanted plants. They are otherwise known as weed killers.
Example:
Simple sodium chlorate (NaClO3) and sodium arsenite (Na3AsO3). Most of the herbicides are toxic to mammals.
Industrial wastes:
Industrial activities have been the biggest contributor to the soil pollution especially the mining Eind manufacturing activities.
Large number of toxic wastes are released from industries. Industrial wastes include cyanides, chromates, acids, alkalis, and metals like mercury, copper, zinc, cadmium and lead etc. These industrial wastes in the soil surface lie for a long time and make it unsuitable for use.
![]()
Question 7.
Explain the various contribution of green chemistry in our day to day life.
Answer:
(1) Dry cleaning of clothes:
Solvents like tetrachloroethylene used in dry cleaning of clothes, pollute the ground water and are carcinogenic. In the place of tetrachloroethylene, liquefied CO2 with suitable detergent is an Alternate solvent used. Liquified CO2 is not harmful to the ground water. Now a days H2O2 used for bleaching clothes in laundry, gives better results and utilizese less water.
(2) Bleaching of paper:
Conventional method of bleaching was done with chlorine. Now a days H2O2 can be used for bleaching paper in presence of catalyst.
(3) Synthesis of chemicals:
Acetaldehyde is now commercially prepared by one step oxidation of ethene in the presence of ionic catalyst in aqueous medium with 90% yield.
CH2 = CH2 + O 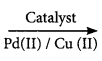 CH3CHO
CH3CHO
Ethylene Acetaldehyde
(4) Instead of petrol, methanol is used as a fuel in automobiles:
Methanol is considered to be less expensive than other commercial fuel and gasoline. During the process of combustion, it provides a higher thermal efficiency and power output because of its high octane rating and high heat vapourization.
(5) Neem based pesticides have been synthesised, which are more safer than the chlorinated hydrocarbons:
Every individual has an important role for preventing pollution and improving our environment. We Eire responsible for environmental protection. Let us begin to save our environment and provide a clean earth for our future generations.