Tamilnadu State Board New Syllabus Samacheer Kalvi 12th Business Maths Guide Pdf Chapter 8 Sampling Techniques and Statistical Inference Ex 8.3 Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 12th Business Maths Solutions Chapter 8 Sampling Techniques and Statistical Inference Ex 8.3
Choose the correct Answer:
Question 1.
A ……………… may be finite or infinite according as the number of observation or items in it is finite or infinite
(a) Population
(b) census
(c) parameter
(d) none of these
Solution:
(a) population
![]()
Question 2.
A …………….. of statistical individuals in a population is called a sample.
(a) Infinite set
(b) finite subset
(c) finite set
(d) entire set
Solution:
(b) finite subset
Question 3.
A finite subset of statistical individuals in a population is called ………………
a) a sample
(b) a population
(c) universe
(d) census
Solution:
(a) a sample
Question 4.
Any statistical measure computed from sample data is known as ……………..
(a) Parameter
(b) random sample
(c) Infinite measure
(d) uncountable
Solution:
(b) random sample
Question 5.
A ………………. is one where each item in the universe has an equal chance of known opportu¬nity of being selected
(a) Parameter
(b) random sample
(c) statistic
(d) entire data
Solution:
(b) random sample
Question 6.
A random sample is a sample selected in such a way that every item in the population has an equal chance of being included
(a) Harper
(b) fisher
(c) karl pearson
(d) Dr. yates
Solution:
(a) Harper
![]()
Question 7.
Which one of the following is probability sampling
(a) Purposive sampling
(b) judgement sampling
(c) sample random sampling
(d) Convenience sampling
Solution:
(c) sample random sampling
Question 8.
In simple random sampling of drawing any unit, the probability of drawing any unit at the draw is ?
(a) \(\frac { n }{N}\)
(b) \(\frac { 1 }{N}\)
(c) \(\frac { N }{n}\)
(d) n
Solution:
(b) \(\frac { 1 }{N}\)
Question 9.
In ……………. the heterogeneous groups divided into homogeneous groups
(a) Non-probability sample
(b) a sample random sample
(c) a stratified random sample
(d) Systematic sample
Solution:
(c) a stratified random sample
Question 10.
Errors in sampling are of
(a) Two types
(b) three types
(c) four types
(d) five types
Solution:
(a) Two types
Question 11.
The method of obtaining the most likely value of the population parameter using statistic is called
(a) estimate
(b) estimate
(c) biased estimate
(d) standard error
Solution:
(d) standard error
![]()
Question 12.
An estimator is a sample statistic used to estimate a
(a) population parameter
(b) biased estimate
(c) sample size
(d) census
Solution:
(a) population parameter
Question 13.
…………… is a relative property, which states that one estimate is efficient relative to another.
(a) efficiency
(b) sufficiency
(c) unbiased
(d) consistency.
Solution:
(a) efficiency
Question 14.
If probability p[|\(\bar { θ }\) – θ|< ∈|< ∈|] 1 → µ as n → α for any positive then \(\bar { θ }\) is said to estimator of θ
(a) efficient
(b) sufficient
(c) unbiased
(d) consistent
Solution:
(d) Consistent
Question 15.
An estimator is said to be ………….. if it contains all the information in the data about the parameter it estimates.
(a) efficient
(b) sufficient
(c) unbiased
(d) consistent
Solution:
(b) sufficient
![]()
Question 16.
An estimate of a population parameter given by two numbers between which the parameter would be expected to lie called an ………….. interval estimate of the parameter
(a) point estimate
(b) interval estimate
(c) standard error
(d) confidence
Solution:
(b) interval estimate
Question 17.
A ……………… is a statement or an assertion about the population parameter
(a) hypothesis
(b) statistic
(c) sample
(d) census
Solution:
(a) hypothesis
Question 18.
Type I error is
(a) Accept H0 when it is true
(b) Accept H0 when it is false
(c) Reject H0 when it is true
(d) Reject H0 when it is false
Solution:
(c) Reject H0 when it is true
Question 19.
Type II error is?
(a) Accept H0 when it is wrong
(b) Accept H0 when it is when it is true
(c) Reject H0 when it is true
(d) Reject H0 when it is false
Solution:
(a) Accept H0 when it is wrong
![]()
Question 20.
The standard error of sample mean is?
(a) \(\frac { σ }{\sqrt{2n}}\)
(b) \(\frac { σ }{n}\)
(c) \(\frac { σ }{√n}\)
(d) \(\frac { σ^2 }{√n}\)
Solution:
(c) \(\frac { σ }{√n}\)


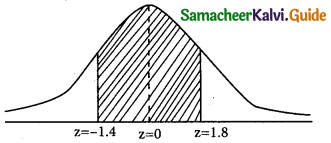
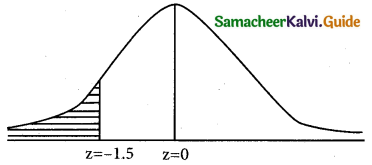
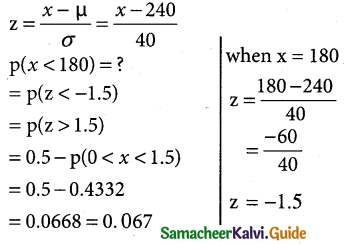
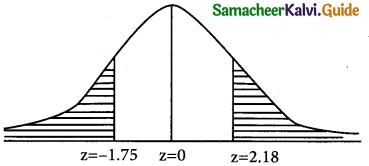
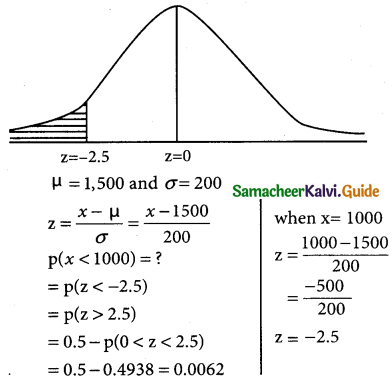
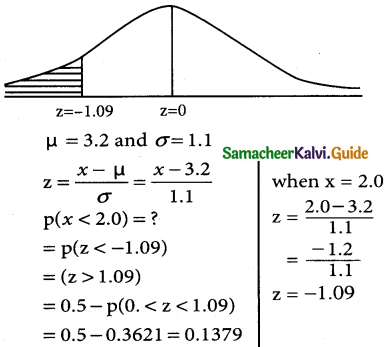
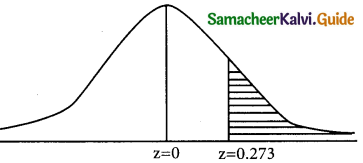
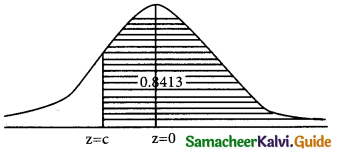
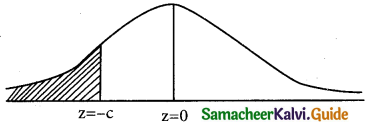
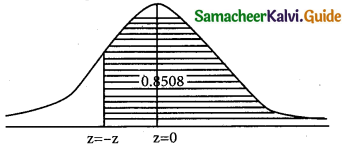
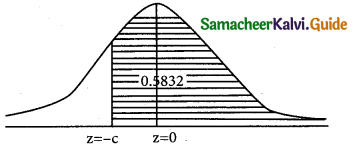

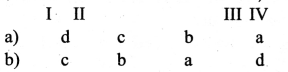


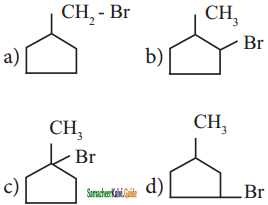
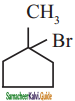
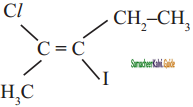
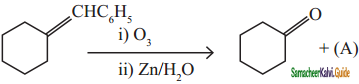
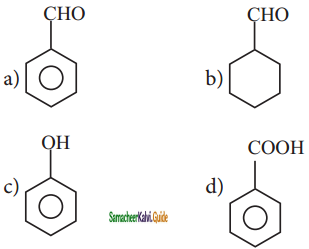
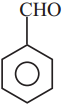
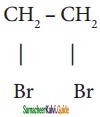
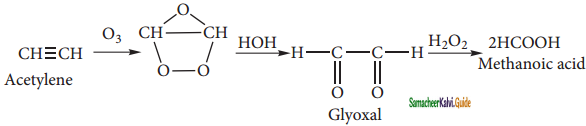
 is
is
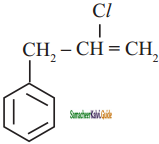
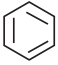
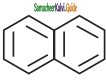
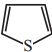
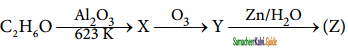
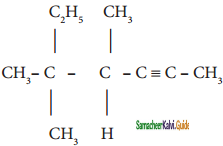
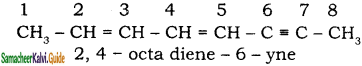
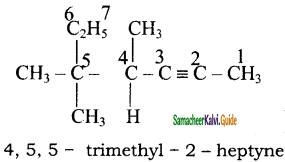
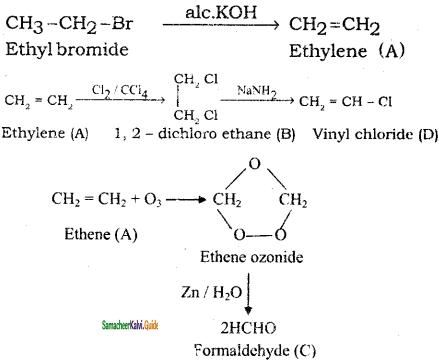
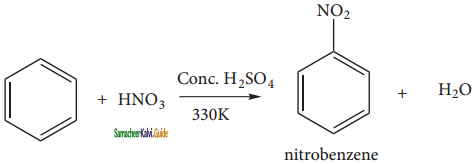
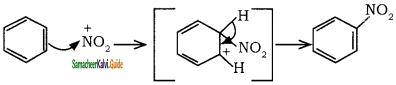
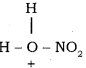 + HSO4–
+ HSO4–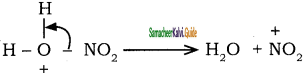
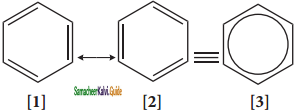
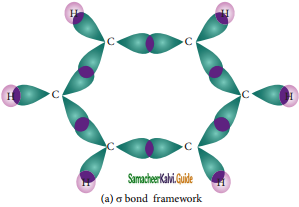
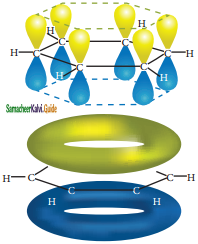
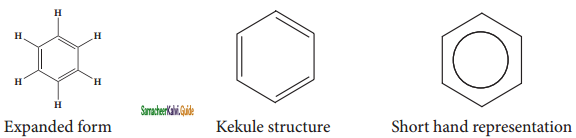
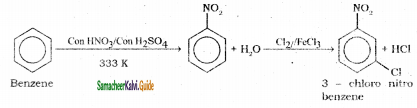
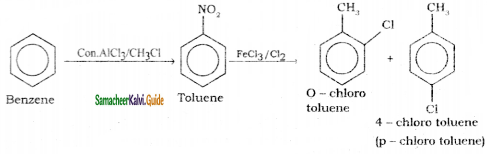
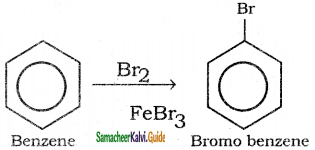
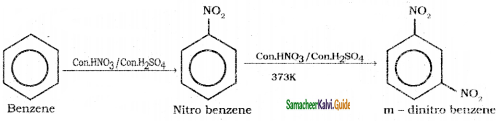
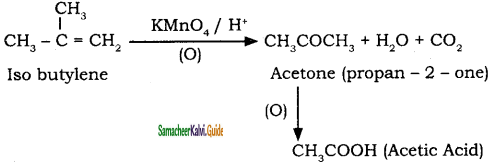
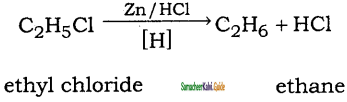
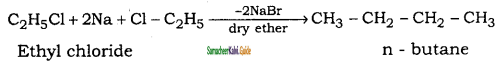
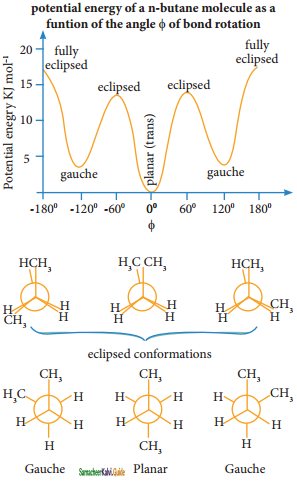
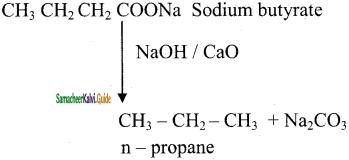
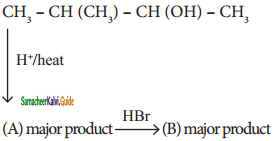
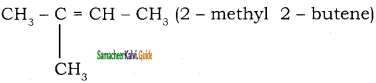
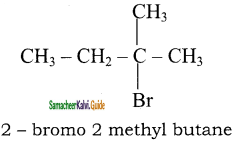
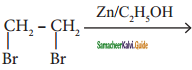
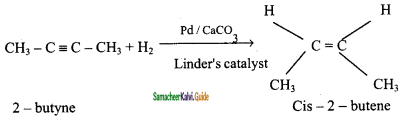
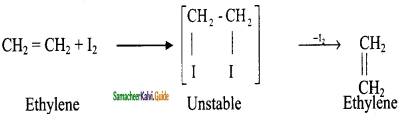
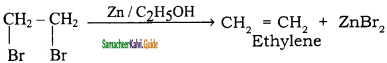
 is
is

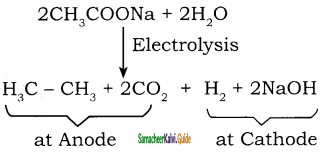
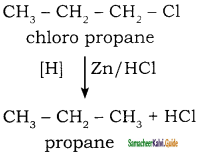
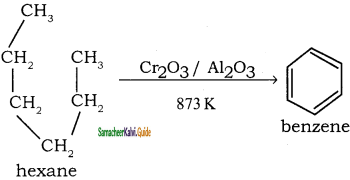
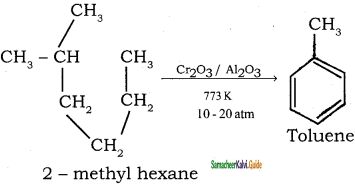
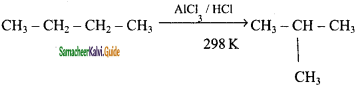
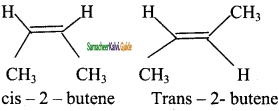
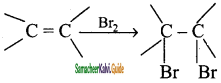
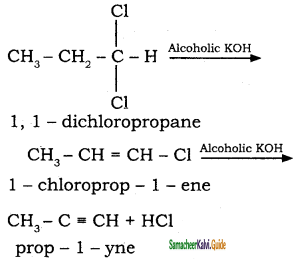
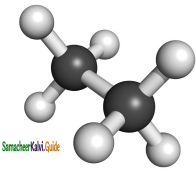
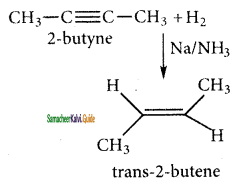
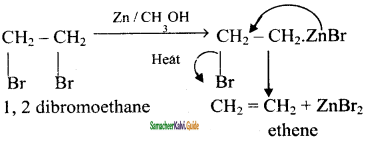
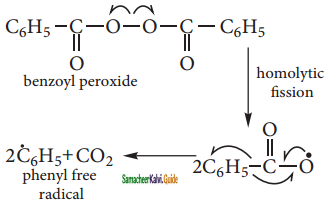
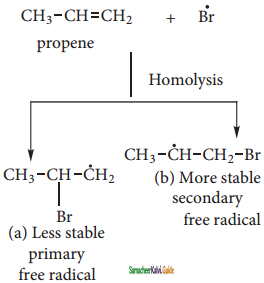
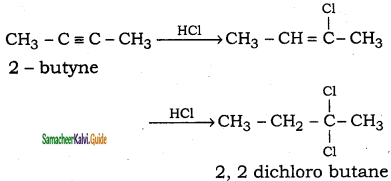
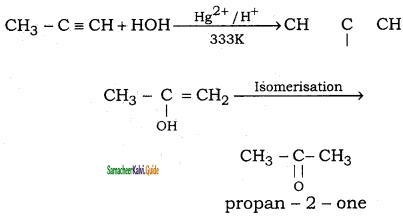
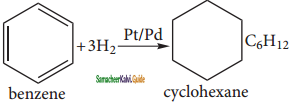
 CH3 – CH3
CH3 – CH3
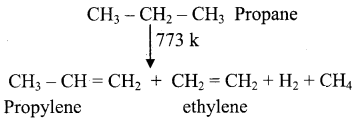
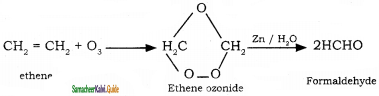
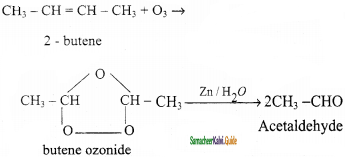
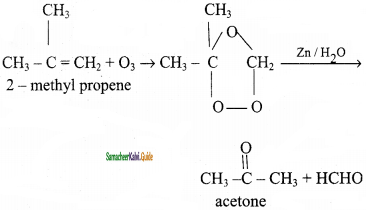
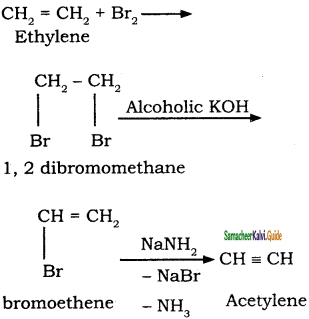
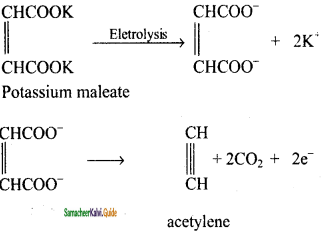
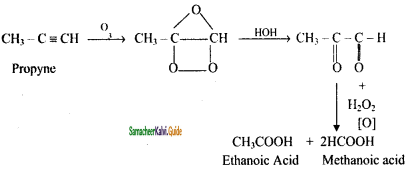
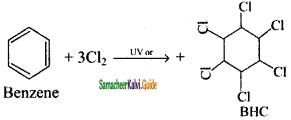
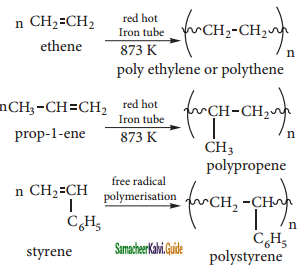
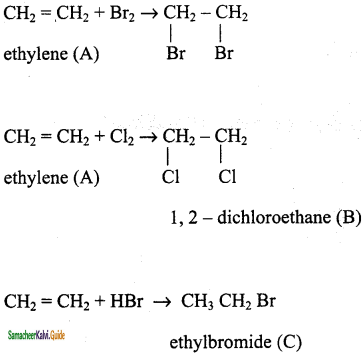
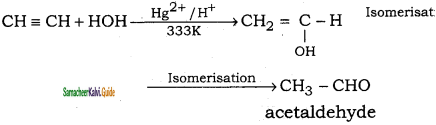
 CH2 = CH2 + 2CH4
CH2 = CH2 + 2CH4
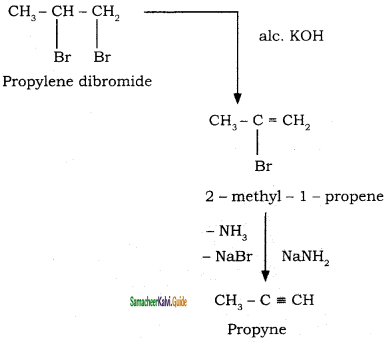
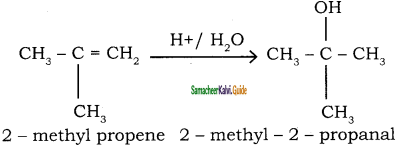
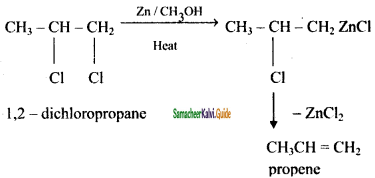
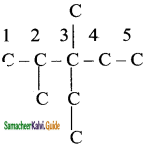
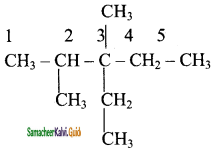
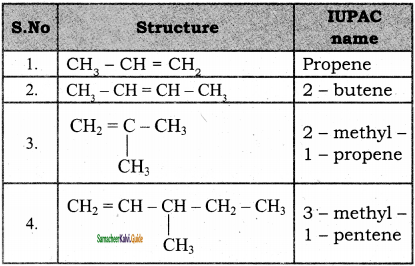
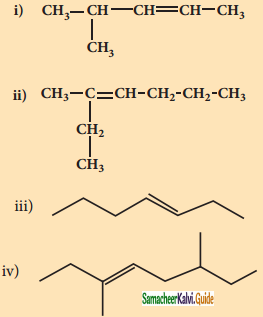
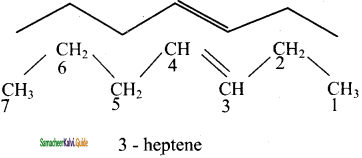
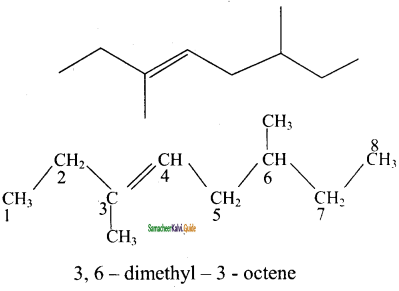
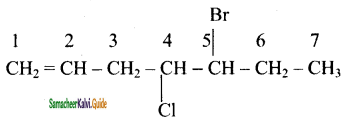
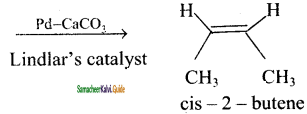
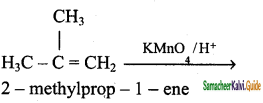
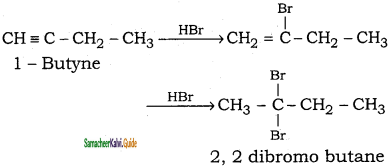
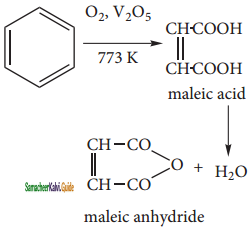
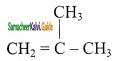 2 – mehyl – 1 – propene
2 – mehyl – 1 – propene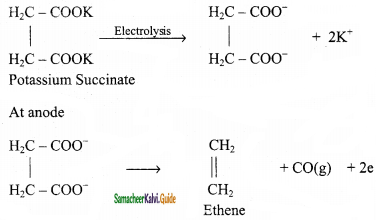
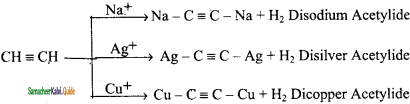
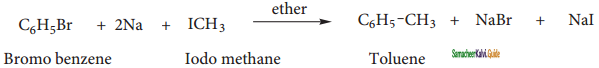

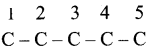
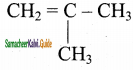
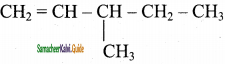
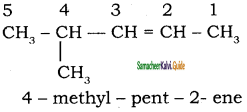
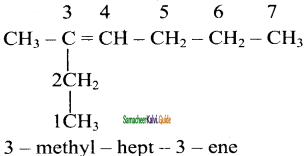
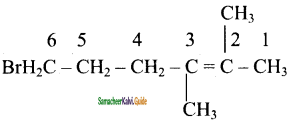
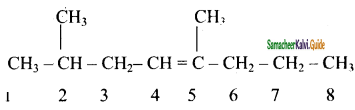
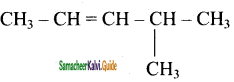
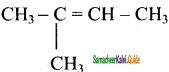
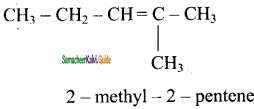
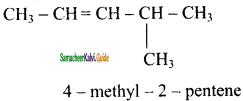

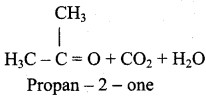

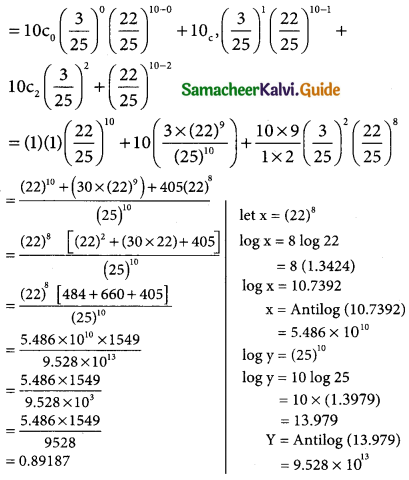
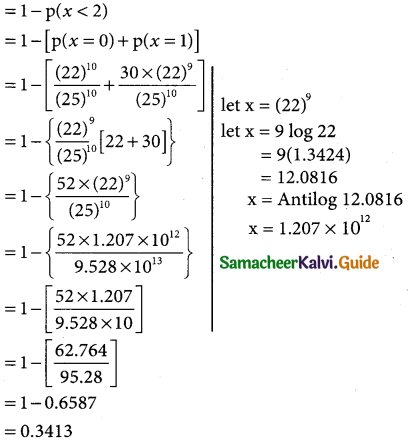
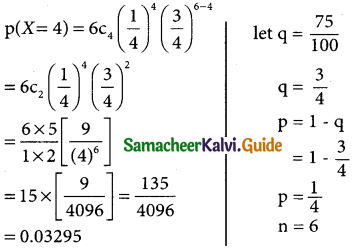
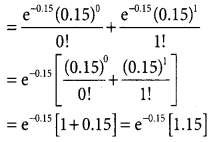
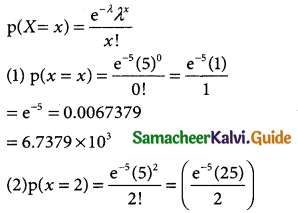



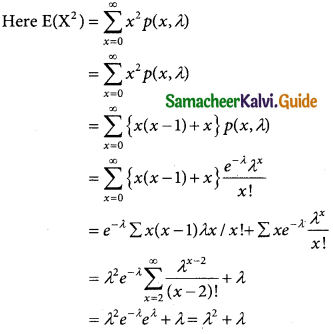




 = 1 – 0.7788 [1 + 0.25]
= 1 – 0.7788 [1 + 0.25]