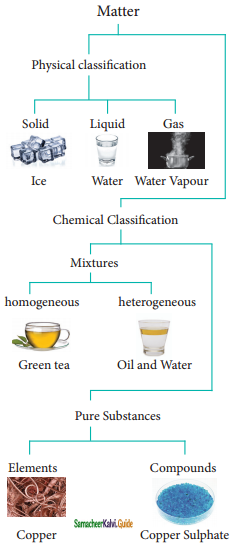
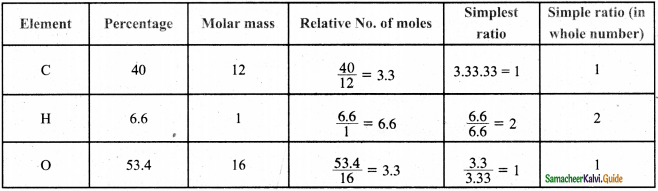
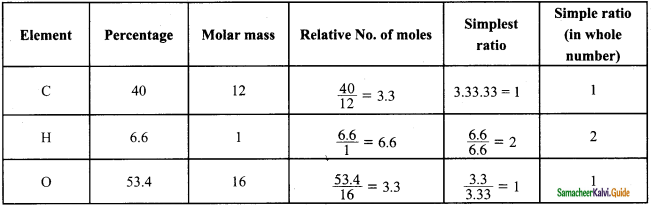
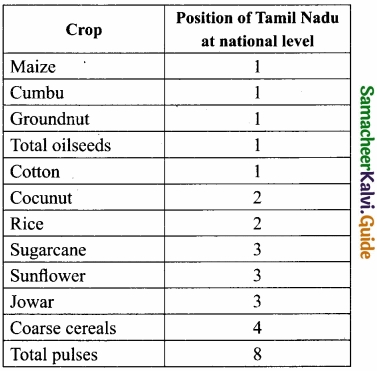
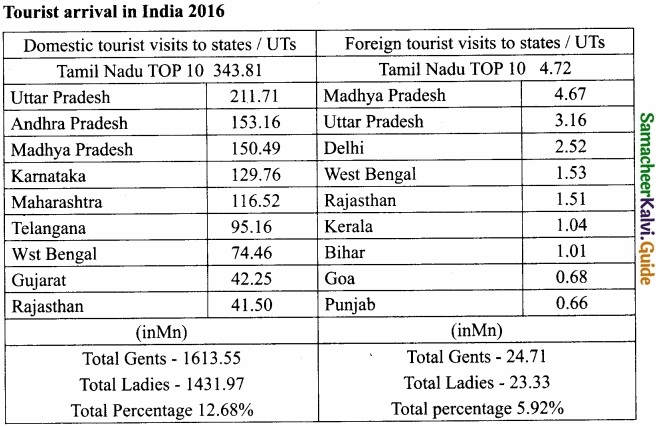
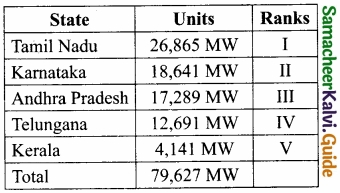
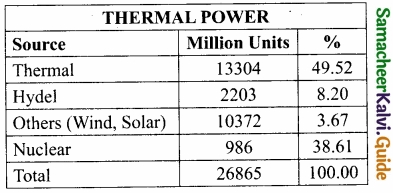
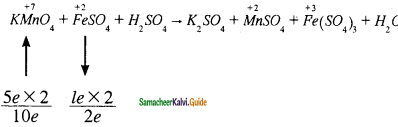Students can Download Samacheer Kalvi 10th Social Science Model Question Paper 4 English Medium Pdf, Samacheer Kalvi 10th Social Science Model Question Papers helps you to revise the complete Tamilnadu State Board New Syllabus, helps students complete homework assignments and to score high marks in board exams.
Tamil Nadu Samacheer Kalvi 10th Social Science Model Question Paper 4 English Medium
General Instructions:
- The question paper comprises of four parts
- You are to attempt all the questions in each part. An internal choice of questions is provided wherever applicable.
- All questions of Part I, II, III, and IV are to be attempted separately.
- Question numbers 1 to 14 in Part I are Multiple Choice Questions of one mark each.
These are to be answered by writing the correct answer along with the corresponding option code and the corresponding answer - Question numbers 15 to 28 in Part II are of two marks each. Any one question should be answered compulsorily.
- Question numbers 29 to 42 in Part III are of five marks each. Any one question should be answered compulsorily.
- Question numbers 43 to 44 in Part IV are of Eight marks each. Draw diagrams wherever necessary.
Time: 3 Hours
Maximum Marks: 100
Part – I
Answer all the questions. Choose the correct answer [14 × 1 = 14]
Question 1.
Who said “imperialism is the highest stage of capitalism”?
(a) Lenin
(b) Marx
(c) Sun Yat-sen
(d) Mao Tsetung
Answer:
(a) Lenin
Question 2.
Who was the Commander-in-Chief responsible for the new military regulations in Vellore Fort?
(a) Col. Fancourt
(b) Major Armstrong
(c) Sir John Cradock
(d) Colonel Agnew
Answer:
(c) Sir John Cradock
Question 3.
Who was the first director of Whanpoa Military Academy?
(a) Sun Yat-Sen
(b) Chiang Kai-shek
(c) Michael Borodin
(d) Chou En Lai
Answer:
(b) Chiang Kai-shek
![]()
Question 4.
Who declared that “Land belongs to God” and collecting rent or tax on it was against divine law?
(a) Titu Mir
(b) Sidhu
(c) Dudu Mian
(d) Shariatullah
Answer:
(c) Dudu Mian
Question 5.
In which session of the Indian National Congress was Non-Cooperation approved?
(a) Bombay
(b) Madras
(c) Lucknow
(d) Nagpur
Answer:
(c) Lucknow
Question 6.
The Southern most point of India is …………………..
(a) Andaman
(b) Kanyakumari
(c) Indira point
(d) Kavaratti
Answer:
(c) Indira point
Question 7.
We wear cotton during …………………..
(a) Summer
(b) Winter
(c) Rainy
(d) Northeast monsoon
Answer:
(a) Summer
Question 8.
Which of the following organization has divided the Indian soils into 8 major groups?
(a) Indian Council of Agricultural Research
(b) Indian Meteorological Department
(c) Soil Survey of India
(d) Indian Institute of Soil Science
Answer:
(a) Indian Council of Agricultural Research
Question 9.
The longitudinal extent of Tamil Nadu is …………………..
(a) 76°18’E to 80°20’E
(b) 76°18’Eto 80°20’W
(c) 86° 18’E to 10°20’E
(d) 86° 18’E to 10°20’W
Answer:
(a) 76°18’E to 80°20’E
![]()
Question 10.
The district with largest mangrove forest cover in Tamil Nadu is …………………..
(a) Ramanathapuram
(b) Nagapattinam
(c) Cuddalore
(d) Theni
Answer:
(c) Cuddalore
Question 11.
If the fundamental rights of Indian citizen are violated, they possess the right to have an access to …………………..
(a) The Parliament
(b) The Attorney General
(c) The President of India
(d) The Supreme Court of India
Answer:
(d) The Supreme Court of India
Question 12.
Non-military issues are …………………..
(a) Energy security
(b) Water security
(c) Pandemics
(d) All the above
Answer:
(d) All the above
Question 13.
Tamil Nadu Integrated Nutrition Programme was started in …………………..
(a) 1980
(b) 1975
(c) 1955
(d) 1985
Answer:
(a) 1980
Question 14.
India is ………………….. large producer in agricultural product.
(a) 1st
(b) 3rd
(c) 4th
(d) 2nd
Answer:
(d) 2nd
Part – II
Answer any 10 questions. Question No. 28 is compulsory. [10 x 2 = 20]
Question 15.
Discuss Mahadev Govind Ranade’s contribution to social reforms.
Answer:
- Mahadev Govind Ranade was a great social reformer. He advocated for inter-caste dining, inter-caste marriage, widow remarriage and improvement of women and depressed classes.
- He founded the Widow Marriage Association, the Poona Sarvajanik Sabha and Decean Education society.
Question 16.
Who were the three prominent dictators of the post World War I?
The three prominent dictators of the post-World War I were Mussolini (Italy), Hitler (Germany) and Franco (Spain).
Question 17.
Identify the Palayams based on the division of east and west.
Answer:
Among the 72 Palayakkarars, there were two blocks namely the eastern and the western Palayams. ‘
- The eastern Palayams were – Sattur, Nagalapuram, Ettayapuram and Panchalam Kurichi.
- The western Palayams were – Uttrumalai, Thalavankottai, Naduvakurichi, Singampatti and Seithur.
Question 18.
What was the conflict between the Swarajists and no-changers?
Answer:
1. In due course of time the Congress got divided into two groups—pro-changers (swarajists) and no-changers. Some of the Congressmen led by Motilal Nehru and C. R. Das wanted to contest the elections and enter the legislature.
2. They argued that the national interest could be promoted by working in the Legislative Councils under Dyarchy and weakening the colonial government from within. These Congressmen were called the pro-changers.
3. On the other hand, no-changers like Vallabhbhai Patel, C Rajagopalachari and other followers of Gandhi wanted to continue non-cooperation with the government.
![]()
Question 19.
Write a short note on ‘Monsoon Wind’.
Answer:
- The word ‘monsoon’ has been derived from the Arabic word ‘Mausim’ which means
‘season’. - These winds appear to blow from southwest for six months and from northeast for another
six months. - In India it is used to refer to the winds which reverse their directions in summer and winter.
Question 20.
What is Multipurpose project?
Answer:
A comprehensive river valley project which serves a number of purposes simultaneously is called a “Multipurpose project”.
Question 21.
Define: Disaster Risk Reduction.
Answer:
The concept and practice of disaster risks through systematic efforts to analyse and manage the causal factors of disasters including thorough reduced exposure to hazards, lessened vulnerability of people and property, wise management of land and environment and improved preparedness for adverse events.
Question 22.
What is the importance of the Governor of a state?
Answer:
- The Governor is the Constitutional head of the State Executive. The administration of a State is carried on in the name of the Governor.
- He directly rules a State when there is the imposition of the President’s rule in the State. He is an integral part of the State legislative.
Question 23.
How do you assess the importance of Chabahar agreement?
Answer:
- A trilateral agreement called the Chabahar Agreement was signed between India, Afghanistan and Iran, which has led to the establishment of transit and transport corridor among three countries using Chabahar port.
- This port is seen as golden gateway for India to access landlocked markets of Afghanistan and Central Asia by passing Pakistan.
Question 24.
Write short note on TRIPs and TRIMs.
Answer:
1. TRIPs – Trade Related aspects of Intellectual Property Rights – is an international legal agreement between all the member nations of the World Trade Organisation (WTO). It sets down minimum standards for the regulation by national Governments of many forms of intellectual property as applied to nationals of other WTO member nations. TRIPs was negotiated at the end of the Uruguay Round of the General Agreement on Tariffs and Trade (GATT) between 1989 and 1990 and is administered by the WTO.
2. TRIMS – Trade Related Investment Measures – The Uruguay Round Agreement on TRIMs referes to certain conditions or restrictions imposed by a Government in respect of foreign investment in the country in order to give adequate provisions for the home industries to develop.
Question 25.
Why should a developing economy diversify out of agriculture?
Answer:
- As an economy grows and incomes increase, consumers tend to spend a lesser share of their income on products from the agricultural sector.
- There are limits to the ability of agriculture to absorb labour due to the declining marginal productivity of land.
- Due to this, there is a need for an economy’s production and employment base to diversify away from agriculture.
Question 26.
During cyclone, how does the Meterological department warn the fishermen?
Answer:
1. During disturbed weather over the seas, the ports likely to be affected are warned by concerned ACWCs / CWCs by advising the port authorities through port warnings to hoist appropriate Storm Warning Signals. The Department also issue “Fleet forecast” for Indian Navy.
2. Coastal Bulletins for Indian coastal areas covering up to 75 km from the coast line and sea area bulletins for the sea areas beyond 75 km. The special warnings are issued for fishermen four times a day in normal weather and every three hourly in accordance with the four stage warning in case of disturbed weather.
3. The general public, the coastal residents and fishermen are warned through state government officials and broadcast of warnings through All India Radio and National Television telecast programmes in national and regional hook up.
4. A sytem of warning dissemination for fishermen through World Space Digital Based radio receiver is being planned.
Question 27.
Mention any three industrial development agencies in Tamil Nadu and their role.
Answer:
SIPCOT: (State Industries Promotion Corporation of Tamil Nadu), 1971 : It was formed in the year 1971 to promote industrial growth in the state by setting up industrial estates.
TANSIDCO: (Tamil Nadu Small Industries Development corporation), 1970 :
TANSIDCO is a state-agency of the state of Tamil Nadu established in the year 1970 to promote small-scale industries in the state. It gives subsidies and provide technical assistance for new firms in the small scale sector.
TIDCO: (TamilNadu Industrial Development Corporation), 1965 :
TIDCO is another government agency to promote industries in the state and to establish industrial estates.
![]()
Question 28.
Write some name of the nutrition programmes in Tamil Nadu.
Answer:
- Purachi Thalaivar M.G.R. Nutrition Meal Programme
- National Programme of Nutritional Support to Primary Education
- General ICDS Projects and World Bank Assisted Integrated Child Development Services
- Pradhan Manthri Gramodaya Yojana Scheme (PMGYS)
- Tamil Nadu Integrated Nutrition Programme
- Mid-Day Meal Programme
Part – III
Answer any 10 questions. Question No. 42 is compulsory. [10 x 5 = 50]
Question 29.
Fill in the blanks:
Answer:
(i) ………………was known as the “Father of modem China”.
(ii) ………………soil is suitable for the cultivation of tea and coffee plants.
(iii) Our tradition and national ethos is to practice………………
(iv) ………………play an important role in the supply of quality goods at responsible rates to common
people.
(v) ………………is the third largest airport in India after Mumbai and Delhi.
Answers
(i) Dr. Sun-yat-sen
(ii) Laterite
(iii) Disarmament
(iv) Consumer co-operatives
(v) Chennai International Airport
Question 30.
Match the following:

Answers:

Question 31.
Match the following:

Answer:

![]()
Question 32.
(a) Distinguish between
(i) Agro based industry and Mineral based industry
(ii) Roadways and Railways
Answer:
(a) (i) Agro based industry and Mineral based industry:
Agro based industry :
- Agro based industries use agricultural products as their basic raw materials
- The major agro based industries of our country are cotton textile industry, Jute industry, sugar industry etc.
- These industries are located near the areas of cultivation.
Mineral based industry :
- Minerals based industries use both metallic minerals and Non-metallic minerals as their raw materials.
- The major minerals based industry, of our country is the iron and steel industry.
- These industries are located either near the coalfields or iron ore mines.
(ii) Roadways and Railways:
Roadways:
- Roadways are cost efficient and the most popular dominant mode of transport.
- They link different part of our country.
- They are used by all sections of the people.
- Construction of roads is less expensive.
- The roads are classified into village roads, District Roads, State Highways, National Highways, Golden Quadrilateral Super Highways, Express Ways, Broad roads and International Highways.
Railways:
- Indian Railways provide the principal mode of transport for freight and passengers.
- It brings people from the farthest comers of our country.
- They promote trade, tourism, education and national integrations.
- Construction of railway is highly expensive.
- Railway lines are classified into three categories namely, Broad gauge, Meter gauge and Narrow gauge.
(b) Give reason: Karur is called the Textile capital of Tamil Nadu.
Answer:
Karur is a well known industrial centre. The city is famous for cottage and handloom textiles.
It exports all kinds of textile items all over the world.
Question 33.
Describe the rise and growth of nationalist politics in South Africa.
Answer:
- There were two major political parties in South Africa – the unionist party which was mainly British, and the South Africa Party which had largely Afrikaners (Boers).
- The first Prime Minister, Botha belonged to the South Africa Party. He ruled in cooperation with the British. But a militant section of the South Africa Party formed the National Party under Herzog.
- Herzog wanted a twin policy of supremacy of whites over Blacks and Afrikaners over British.
- In the 1920 elections, the National Party gained forty-four seats. The South Africa Party, now led by
- Smuts, secured forty-one seats.
- The British dominated Unionist Party now merged with the South Africa Party. This gave Smuts a majority over the militant Afrikaner-controlled National Party.
Question 34.
Critically examine the Civil Disobedience Movement as the typical example of Gandhian movement.
Answer:
1. Programmes such as no-base campaigns caught the imagination of the peasants. Gandhi announced a no-tax campaign in Bardoli in February 1922. These movements greatly enhanced Gandhi’s reputation as a national leader, especially the peasants.
2. Gandhi made a nation-wide tour. Wherever he visited there was a bonfire of foreign clothes. Thousands left government jobs, students gave up their studies in large numbers and the lawyers gave up thriving practices.
3. Boycott of British goods and institutions were effective. The boycott of the Prince of Wales’ visit to India was successful. During this boycott trade unions and workers participated actively.
4. However, Gandhi suddenly withdrew the movement because of the Chauri Chaura incident. On 5 February 1922 a procession of the nationalists in Chauri Chaura, a village near Gorakhpur in present-day Uttar Pradesh provoked by the police turned violent.
5. The mob burnt the police station 22 policemen lost their lives. Gandhi immediately withdrew the movement. Gandhi was arrested and was released only in 1924.
![]()
Question 35.
What is urbanization? Explain its impacts.
Answer:
The process of society’s transformation from rural to urban is known as urbanization. The level of urbanization of a place is assessed based on the size of population of the towns and cities and the proportion of population engaged in non agricultural sectors. These two are closely linked to the process of industrialization and expansion of the secondary and tertiary sectors of economy.
Impacts of urbanization:
- Urbanization and population concentration go hand – in – hand and are closely related to
each other. A rapid rate of urbanization in a society is taken as an indicator of its economic development. ‘ - Urbanization is increasing rapidly in the developing countries including India.
- Rural to urban migration leads to population explosion in urban areas. Metropolitan cities like Mumbai, Kolkatta and Delhi have more population than that can accommodate.
The following are the major problem of urbanization in India:
- It creates urban sprawl.
- It makes overcrowding in urban centres.
- It leads to shortage of houses in urban areas.
- It leads to the formation of slums.
- It increases traffic congestion in cities. ‘
- It creates water scarcity in cities. ,
- It creates drainage problems.
- It poses the problem of solid waste management.
- It increases the rate of crime.
Question 36.
Explain the characteristic features of summer and winter seasons of Tamil Nadu.
Answer:
Summer Seasons:
- The apparent migration of the sun towards north during March, April and May results in the reception of vertical sun’s rays by South India. Thus there is a steady rise in temperature from the equator.
- Hence, Tamil Nadu located to the south of Tropic of Cancer, experiences high temperature. Generally the temperature varies from 30°C to more than 40°C.
- During this season particularly in the month of May, southern part of the state receives some rainfall from pre-monsoon showers (Mango/Blossom showers) and some parts experience convectional rainfall.
Winter Seasons:
- During January and F ebruary, the vertical ray s of the sun fal 1 between the Tropic of Capricorn and the Equator. Hence, Tamil Nadu and India on the whole receive slanting rays from the sun. So, the weather is slightly cooler during these months.
- The difference between summer and winter temperature is not very high. Winter temperature in Tamil Nadu varies from 15°C to 25°C. However, in the hill stations, the winter temperature drops below 5°C occasionally.
- Some valleys in the Nilgiris record even 0°C. This drop in temperature leads to the formation of thick mist and frost. This season is practically dry.
Question 37.
Explain the Jurisdiction and powers of the Supreme Court of India.
Answer:
The Supreme Court is the guardian of the constitution. He performs the following functions.
1. Original Jurisdiction :- The cases which are brought directly in the first instance to the Supreme Court come under original jurisdiction. These may be – (a) dispute between the government of India and one or more States, (b) dispute between two or more States and (c) the cases involving fundamental rights come under the jurisdiction of the Supreme Court.
2. Appellate Jurisdiction :- The Supreme Court is the final appellate Court in the country. It hears appeals against the decisions of High Court in “civil, criminal and constitutional” cases with a certificate from the High Court that it is fit to appeal in the Supreme Court.
3. Advisory Jurisdiction :- The Constitution confers on the President the power to refer to the Supreme Court any question of law or fact which in his opinion is of public importance.
4. The Law declared by the Supreme Court is binding on all courts within the territory of India.
5. The Supreme Court also enjoys the power of judicial review.
Question 38.
Trace the reason for the formation of BR1CS and write its objectives.
Answer:
Reason for the formation of BRICS:
- To be an alternative to World Bank and IMF to challenge U.S. supremacy.
- To provide self-owned and self-managed organisations to carry out developmental and economical plans in its member nations.
Objectives of BRICS :
- To achieve regional development
- It acts as a bridge between developed and developing countries
- To contribute extensively to development of humanity
- To establish a more equitable and fair world
- Boost intra BRICS trade in their local currencies to increase trade cooperation and cope with the current international financial crisis.
- To promote the technological information exchange among the member states.
- To enhance inclusive economic growth that will lead to an increase in the creation of jobs, fight against poverty and accelerate the economic transformation of members.
![]()
Question 39.
Write about the composition of GDP in India.
Answer:
Indian economy is broadly divided into three sectors which contribute to the GDP –
- Primary Sector – It includes agriculture based allied activities, production of raw materials such as cattle farm, fishing, mining, forestry etc. It is also called agricultural sector.
- Secondary Sectqr – It includes industries that produce a finished, usable product or are involved in construction. This sector generally takes the output of the primary sector and manufactures finished goods. It is also called industrial sector.
- Tertiary Sector – It is known as service sector and includes transport, insurance, banking, trade, education, health care etc.
Question 40.
Elaborate the Public Distribution System.
Answer:
1. The increase in food grain production need not result in increase in access to food for all. Given the unequal distribution of income and the level of poverty that persists in Indian economy, the government took steps to distribute food grains at subsidised rates through the Public Distribution System (PDS).
2. The nature, scope and functioning of PDS varies from state to state. While Tamil Nadu has adopted an ‘Universal’ PDS, the rest of the states in India had a ‘Targeted’ PDS. Under universal PDS all the family ration card holders are entitled to the supplies from PDS.
3. In the targeted PDS, the beneficiaries are identified based on certain criteria and given their entitlements, leaving out the rest. Both the Union and the State governments subsidised the supplies distributed through PDS. The level and quantum of subsidy also varied across states.
Question 41.
Draw a time line for the following:
Write any five important events between 1870-1907
Answer:
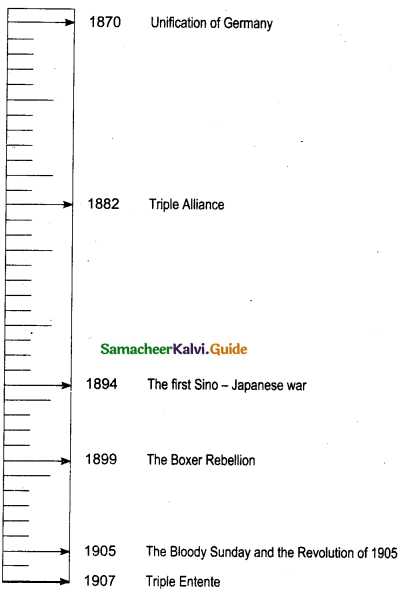
![]()
Question 42.
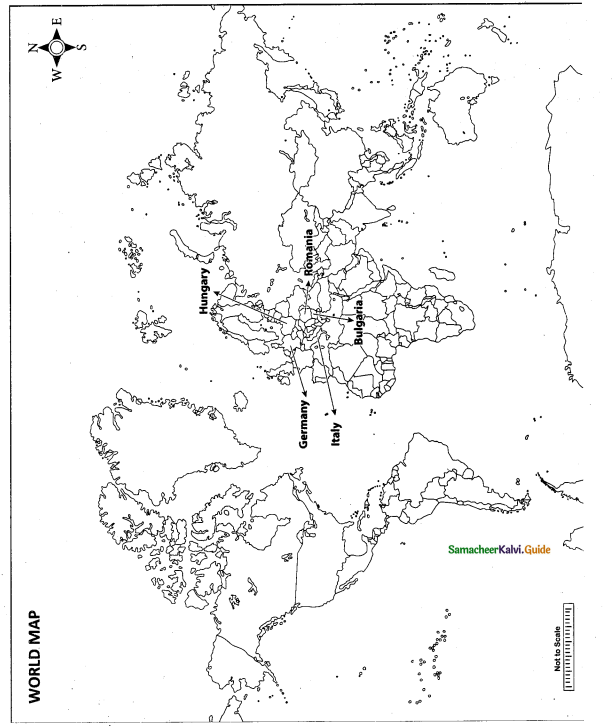
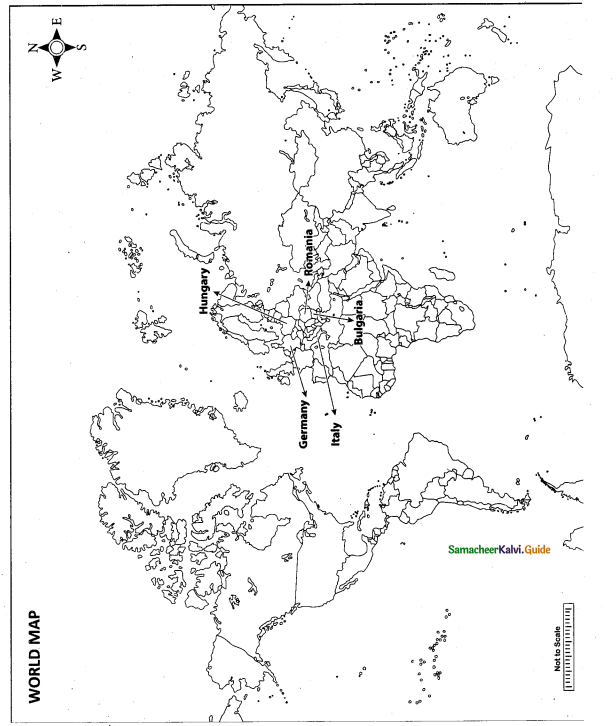
Mark the following places on the world map.
(i)Germany
(ii) Hungary
(iii) Romania
(iv) Bulgaria
(v) Italy
Answer:
Part – IV
Answer both questions. [2 x 8 = 16]
Question 43.
(a) General Assembly and Security Council
(i) List the permanent member countries of the Security Council.
(ii) What is the Holocaust?
(iii) Who was the Chairperson of the UN Commission on Human Rights?
(iv) What is meant by veto?
Answer:
(a) General Assembly and Security Council:
(i) The United States, Britain, France, Russia and China.
(ii) The word ‘holocaust’ is used to describe the genocide of nearly six million Jews by the Germans during the Second World War.
(iii) The widow of US President Franklin Roosevelt was the chairperson of the UN Commission on Human Rights.
(iv) A veto is the power to unilaterally stop an official action, especially the enactment of legislation.
(b) Organs of the EU
(i) Which is the Legislative body of the EU?
(ii) Where is the seat of the Court of Justice?
(iii) What is the function of the European commission?
(iv) Who is responsible for the foreign exchange operation?
Answer:
(b) Organs of the EU
(i) The European Parliament
(ii) Luxemburg
(iii) It is responsible for initiating legislation and the day to day running of the EU.
It drafts proposals for New European laws and presents to the European parliament and the council.
(iv) The European central bank.
[OR]
(c) Dheeran Chinnamalai
(i) When was Dheeran Chinnamalai born?
(ii) How did he earn the title “Chinnamalai”?
(iii) Name the Diwan of Tipu Sultan.
(iv) Why and where was he hanged to death?
(c) Dheeran Chinnamalai
(i) Dheeran Chinnamalai was bom in 1756 in the Mandradiar royal family of Palayakottai.
(ii) Once when Tipu’s diwan Mohammed Ali was returning to Mysore with the tax money, Theerthagiri blocked his way and took back all the tax money. He let Mohammed Ali go by instructing him to tell his Sultan that ‘Chinnamalai; who is between Sivamalai and Chinnamalai, was the one who took away taxes. Thus, he gained the name ‘Dheeran Chennamalai’.
(iii) Mohammed Ali
(iv) He was hanged at the top of the Sankagiri Fort on 31 July 1805 because he refused to accept the rule of the British.
(d) Indian National Congress:
(i) What were the techniques adopted by the Congress to get its grievances redressed?
(ii) What do you know of Lal-Bal-Pal triumvirate?
(iii) Where was the first session of Indian National Congress held?
(iv) How did the British respond to the Swadeshi Movement?
Answer:
(d) Indian National Congress:
(i)The techniques adopted by the Congress to get its grievances redressed included appeals, petition and delegation to Britain.
(ii) Lala Lajpat Rai of Punjab, Bal Gangadhar Tilak of Maharashtra and Bipin Chandra Pal of Bengal were three prominent leaders during the Swadeshi period. They are often referred to as Lal-Bal-Pal triumvirate.
(iii) The first session of Indian National Congress was held at Bombay.
(iv) The British brutally crushed the Swadeshi movement by arresting prominent leaders and putting them into the prison.
![]()
Question 44.
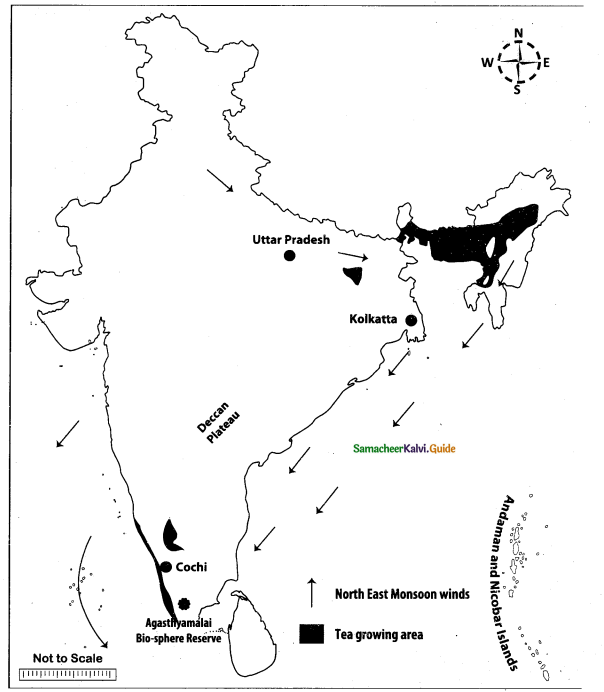
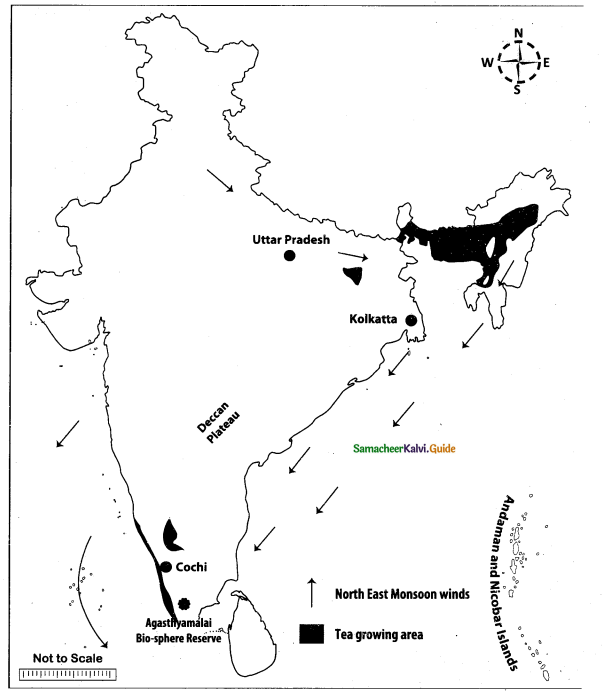
Mark the following places on the given outline map of India.
(i) Uttar Pradesh
(ii) North east monsoon
(iii) Agasthiyamalai bio-sphere reserve
(iv) Kolkata
(v) Tea growing area
(vi) Deccan plateau
(vii) Andaman and Nicobar
(viii) Cochin
Answer:
[OR]
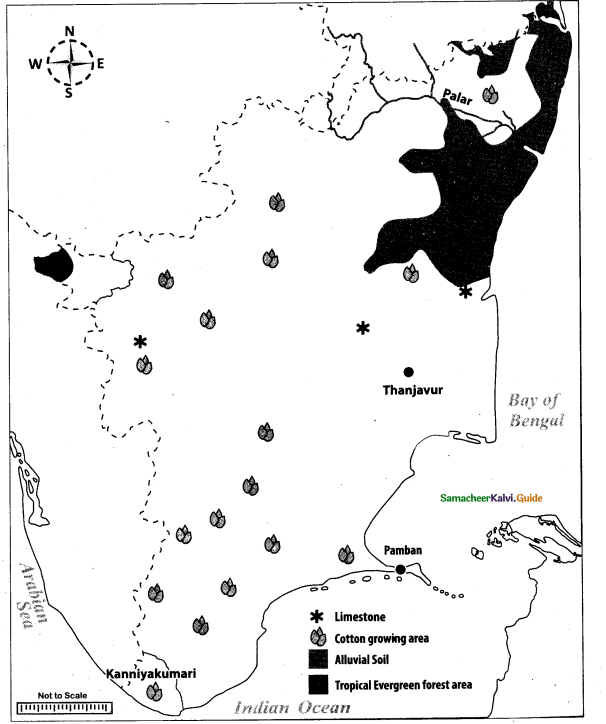
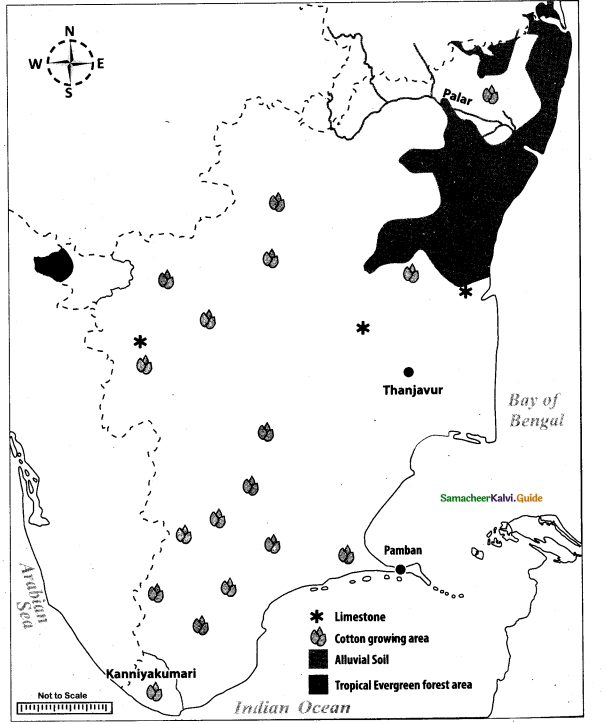
Mark the following places on the given outline map of Tamil Nadu:
(i) Palar
(ii) Tropical Evergreen forest area
(iii) Alluvial soil
(iv) Thanjavur
(v) Kanniyakumari
(vi) Cotton growing area
(vii) Limestone area
(viii) Pamban
Answer:
Map for Q. 42
(i) Germany
(ii) Hungary
(iii) Romania
(iv) Bulgaria
(v) Italy
Answer:
Map for Q. 44
(i) Uttar Pradesh
(ii) North east monsoon
(iii) Agasthiyamalai bio-sphere reserve
(iv) Kolkata
(v) Tea growing area
(vi) Deccan plateau
(vii) Andaman and Nicobar
(viii) Cochin
Answer:
Map for Q. 44
(i) Palar
(ii) Tropical Evergreen forest area
(iii) Alluvial soil
(iv) Thanjavur
(v) Kanniyakumari
(vi) Cotton growing area
(vii) Limestone area
(viii) Pamban
Answer: