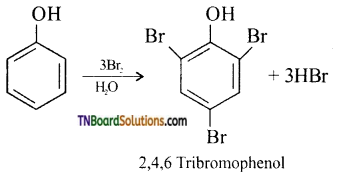
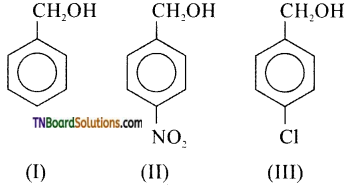
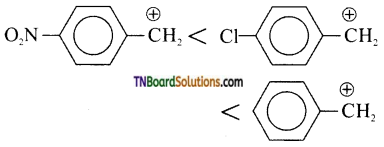
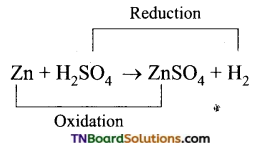
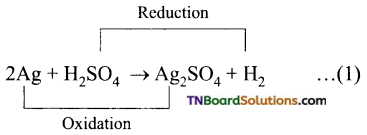
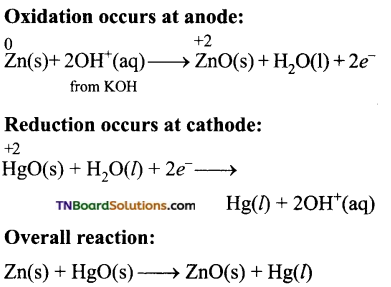
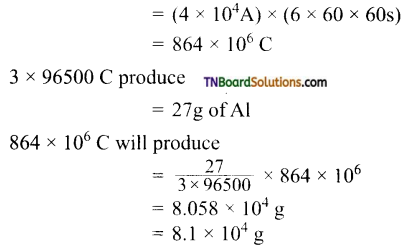
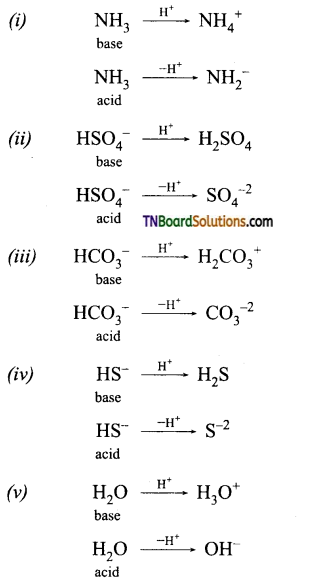
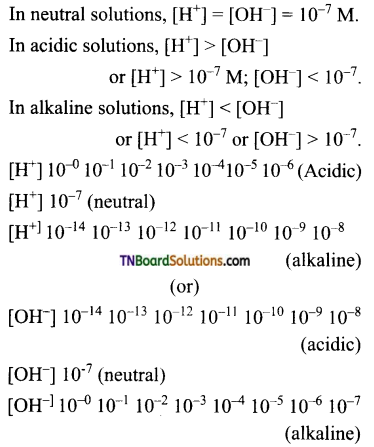
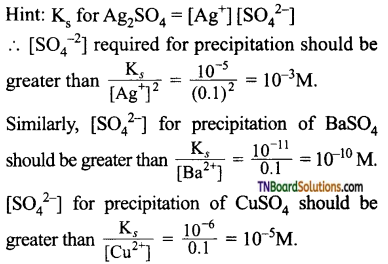
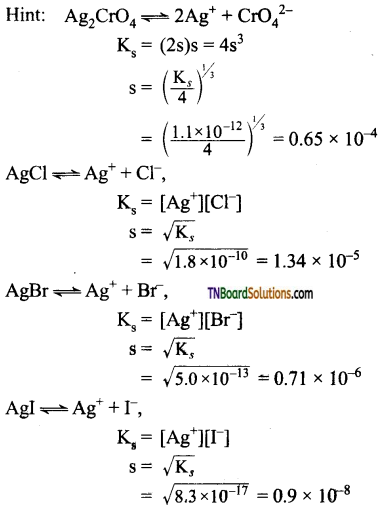
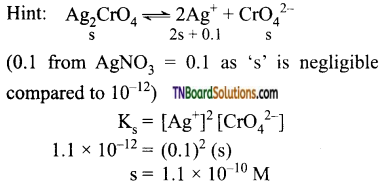
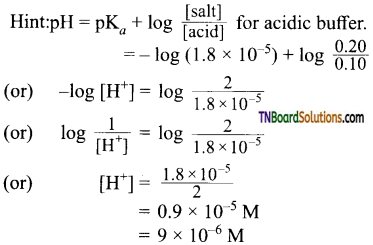
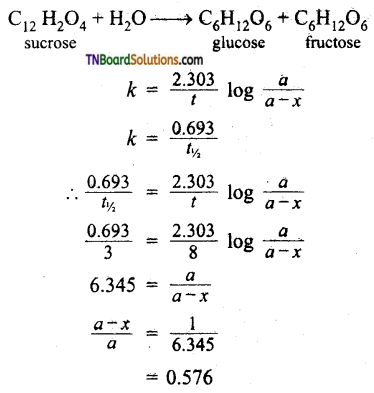
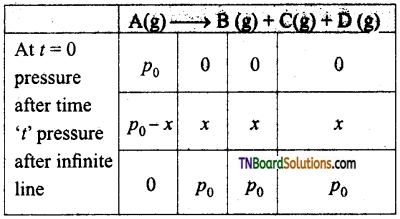
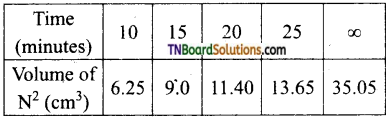
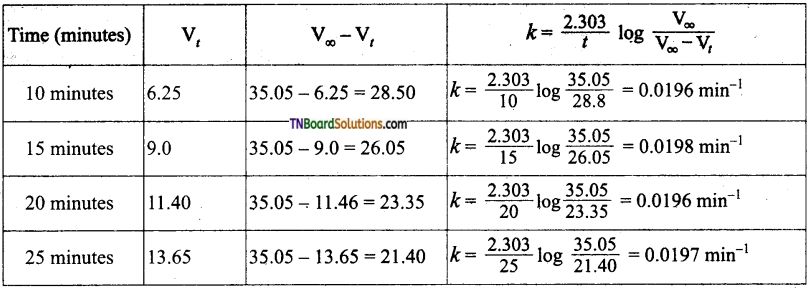
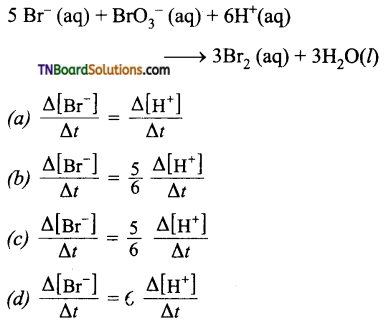
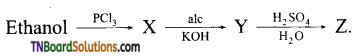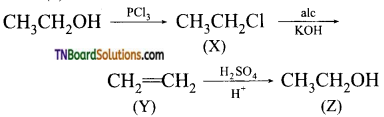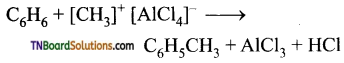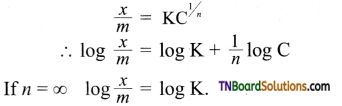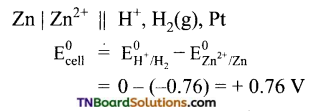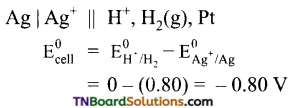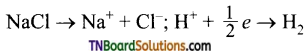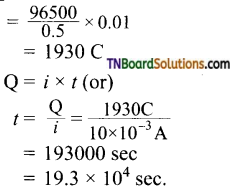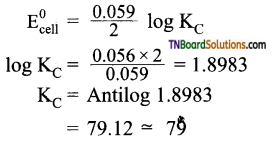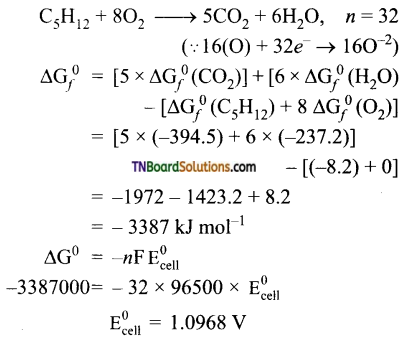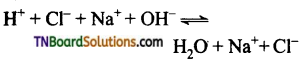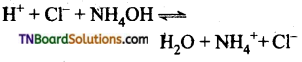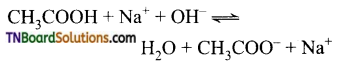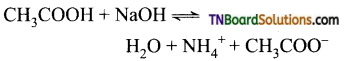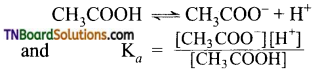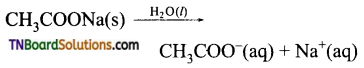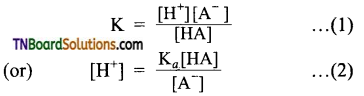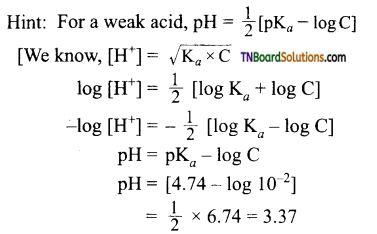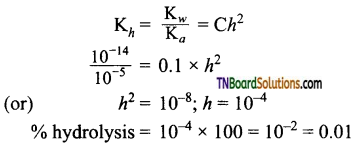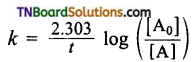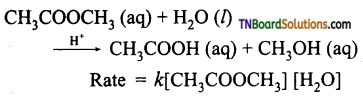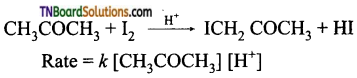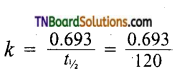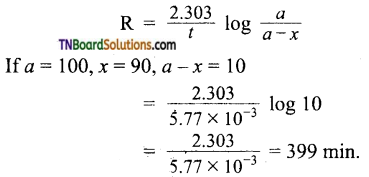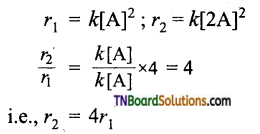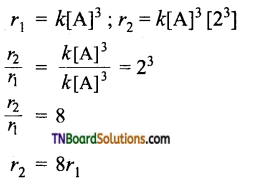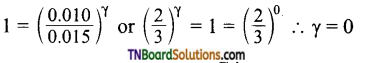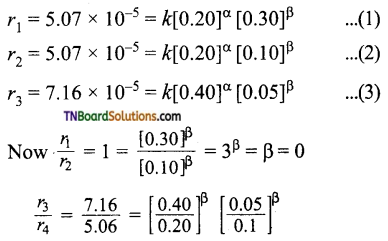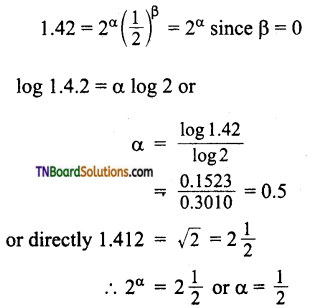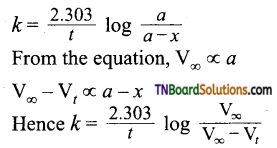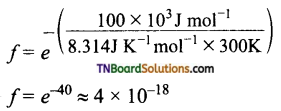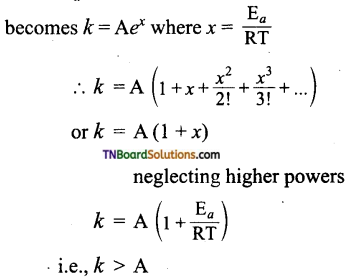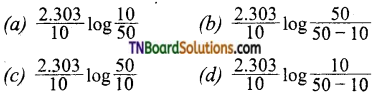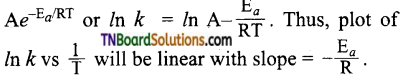Students get through the TN Board 11th Commerce Important Questions Chapter 28 Balance of Trade and Balance of Payments which is useful for their exam preparation.
TN State Board 11th Commerce Important Questions Chapter 28 Balance of Trade and Balance of Payments
Very short answer questions
Question 1.
How many components in the capital Account?
Answer:
- Private capital.
- Banking capital.
- Official capital.
Question 2.
What is visible trade?
Answer:
Import and Export of goods.
![]()
Question 3.
What is invisible trade?
Answer:
Invisible trade service items like banking, shipping, insurance, travel and transportation.
Question 4.
Explain the unfavourable balance of trade.
Answer:
In one country the import of goods is more than exports is called the unfavourable balance of trade.
Short answer questions
Question 1.
What is the net result revealed by BOP?
Answer:
A Balance of Payment deficit points to the fact that the country’s import is more than the export. This situation forces the country to borrow from other countries to pay for its imports. It creates economic development in the short term. It is just similar to taking an educational loan from the bank to pay school fees of children expecting their salary in the future which would help repay the loan.
Question 2.
Explain any three features of the balance of payments.
Answer:
- It is a systematic record of all economic transactions between one country and certain other countries of the world.
- It is prepared for a period of three months or twelve months, i.e., usually 12 months.
- It contains all receipts and payments both visible and invisible.
![]()
Question 3.
What are the debit items shown in the currents account?
Answer:
Goods Import, Invisible Import.
- Transport services purchased from foreign countries.
- Banking services purchased from foreign countries.
- Insurance services purchased from foreign countries.
- Visit of our tourists to foreign countries.
- Other services purchased from foreign countries.
- Interest paid on loan in the home country.
Long answer questions
Question 1.
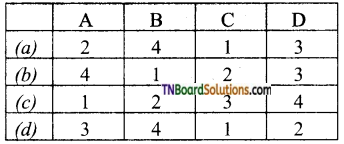
Difference between credit items and debit items in the current account.
Answer:
| Credit Items | Debit Items |
| Goods Export(visible) | Goods Import |
| Invisible-Exports | Invisible Imports |
| Transport service sold abroad | Transport services purchased from foreign countries |
| Banking service sold abroad | Banking services purchased from foreign countries |
| Insurance service sold abroad | Insurance services purchased from foreign countries |
| Income received on loan and investment made in foreign countries | Visit of our tourists to foreign countries |
| Expenses incurred by foreign tourists in India | Other services purchased from foreign countries |
| Interest paid on loan in the home country |
![]()
For Future Learning
1. Impact of Balance of Payments and Trade.
Answer:
BOP shows a favourable or surplus position when the total receipts from foreign countries exceed the total payments to foreign countries are less than the payments to foreign countries. BOP is said to be unfavourable or in deficit.
BOP position shows the economic health of the nation just like the thermometer indicates the temperature of the human body. Favourable BOP indicates economic prosperity while an unfavourable balance of payments shows the economic weakness of a country.
2. Necessary for Global Village concept.
Answer:
- The current account.
- The financial account.
- The capital account.
Together with included in the overall balance of payments.
It includes total inflows and outflows for a given nation.
The current account states that the balance of trade means the purchase and sale of goods and services.
For Own Thinking
1. Balance of Payment is key to economic development.
Answer:
Balance of payments help in framing monetary, fiscal and trade policies of a country government keenly observes the balance of payment position of its important trade – partners in its making policy decisions. It reveals whether a country produces enough economic output to pay for its growth.
2. Importance of BOP and BOT.
Answer:
BOP: Balance of payment refers to a systematic record of all economic transactions between the residents of one country and the residents of foreign countries during a particular period of time, eg: One year contains a classified record of all receipts and payments arising from goods exported.
BOT: Balance of trade denotes the difference between the value of import and the value of export during a year. If the export of a country exceeds its imports, It shows a favourable balance of trade. If the import exceeds the exports, it shows the unfavourable balance of trade.
![]()
Multiple-choice questions
1. Balance of payments refers to a systematic record of all economic transaction between the period:
(a) One year
(b) Two years
(c) Three years
(d) Five years
Answer:
(a) One year
2. Balance payments it is prepared for a period of three months or ………. months.
(a) 9
(b) 6
(c) 12
(d) 1
Answer:
(c) 12
3. Balance of payment includes all economic transactions both recorded on:
(a) Current account
(b) Fixed account
(c) Savings account
(d) Recurring account
Answer:
(a) Current account
4. Balance of payment indicates a country’s position in:
(a) Home trade
(b) Foreign trade
(c) itinerant trade
(d) Small scale fixed retail trade
Answer:
(b) Foreign trade
![]()
5. If the export of country exceeds its ………. shows the favourable balance of trade.
(a) import
(b) export
(c) entrepot
(d) home trade
Answer:
(a) import
6. The balance of payments consists of ……….. components.
(a) one
(b) two
(c) three
(d) four
Answer:
(d) four
7. The current account balance includes:
(a) one item
(b) two items
(c) three items
(d) four items
Answer:
(b) two items
8. Private capital consists of foreign investment ……… and foreign currency deposits.
(a) short term
(b) long term
(c) one year
(d) more than 5 years
Answer:
(b) long term
9. The current account balance invisible service:
(a) banking
(b) trade
(c) advertisement
(d) consumer
Answer:
(a) banking
![]()
10. The current account bank visible trade:
(a) small scale fixed trade
(b) home trade
(c) import & export
(d) large scale trade
Answer:
(c) import & export
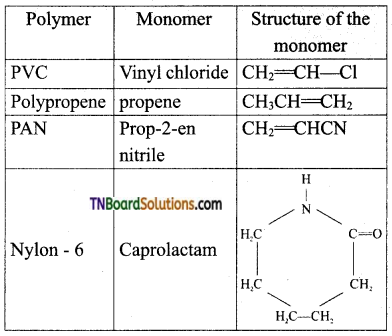
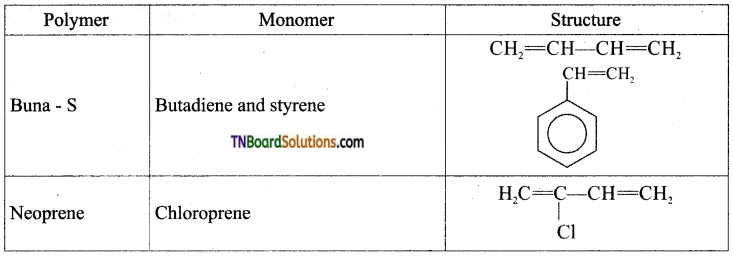
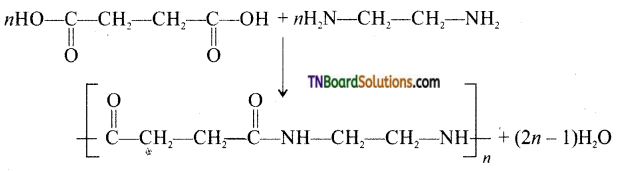
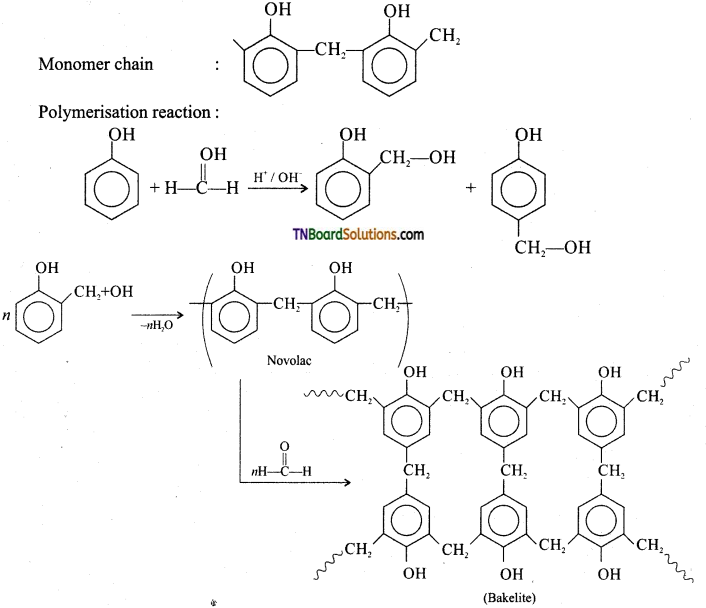
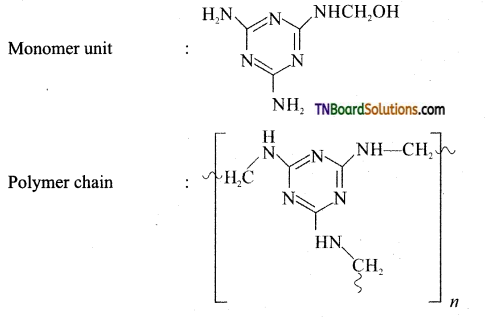
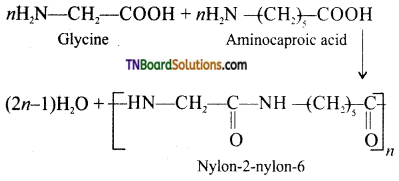
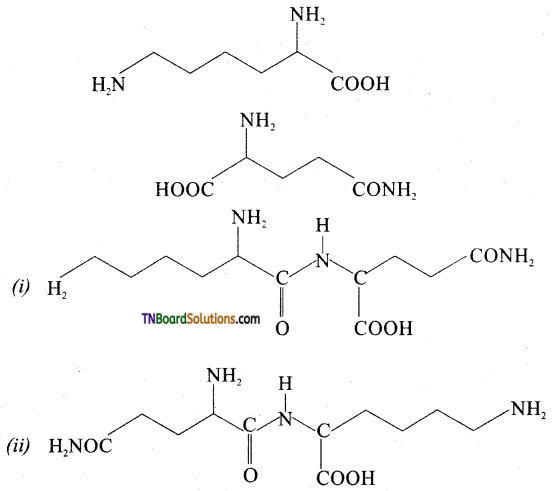
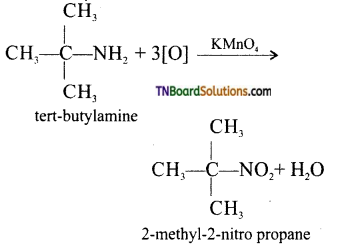
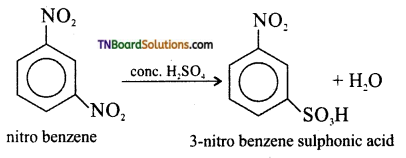
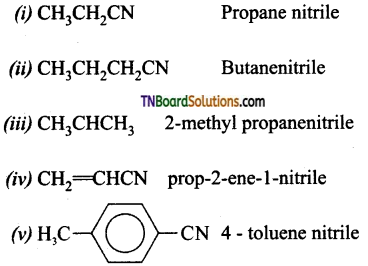
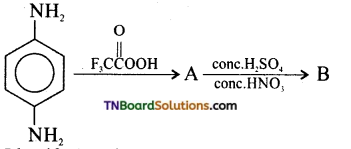
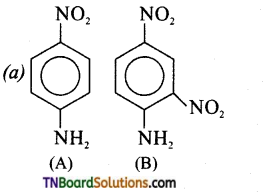
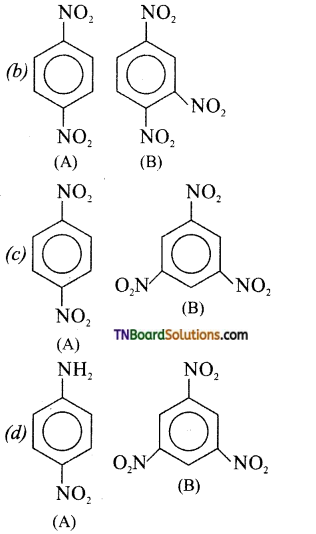
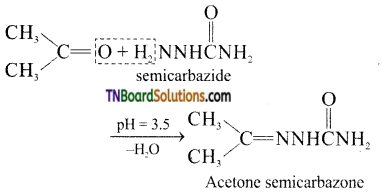
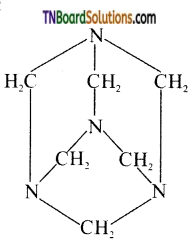
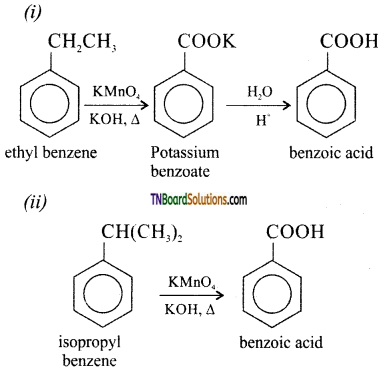
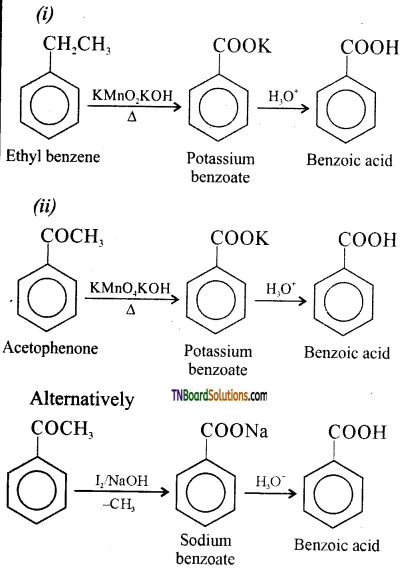
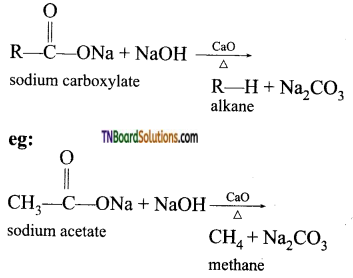
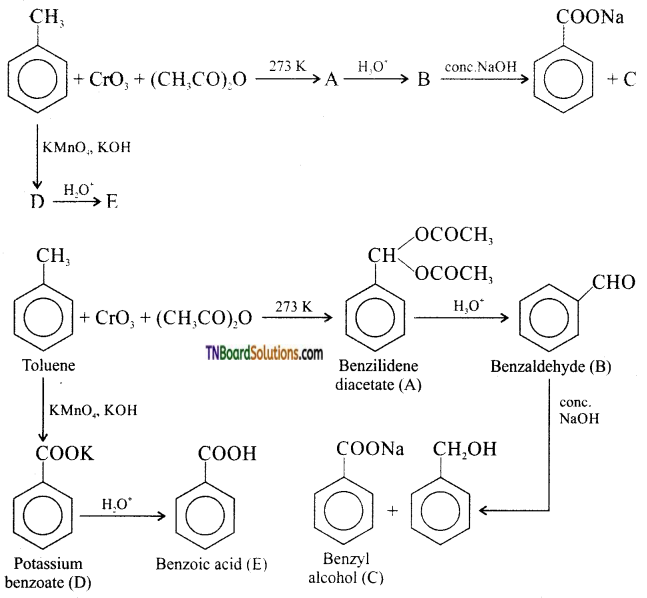
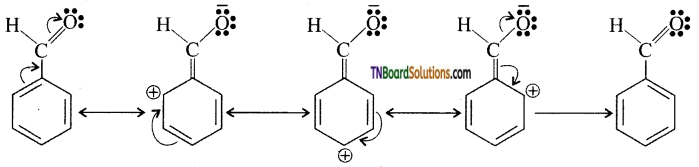
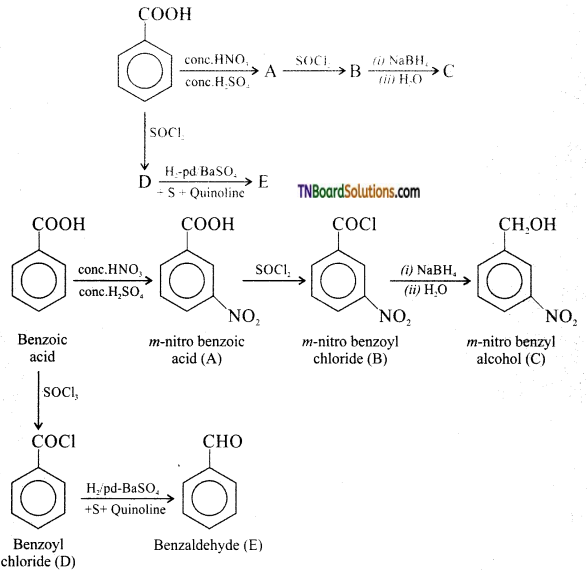
 which is derived from caprolactam.
which is derived from caprolactam.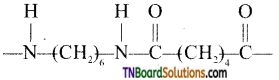
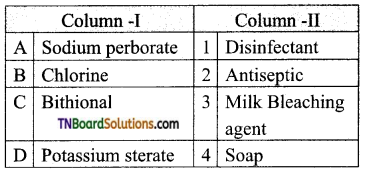
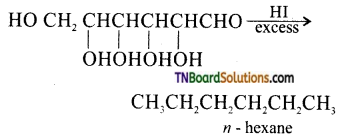
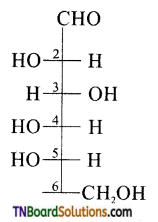
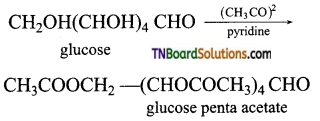
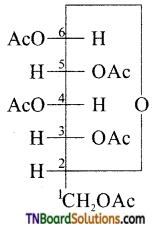
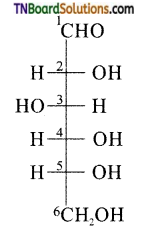
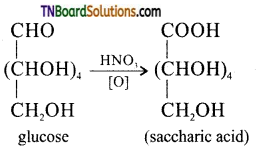
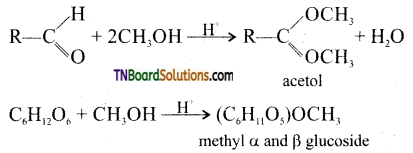
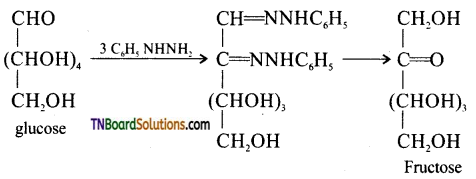
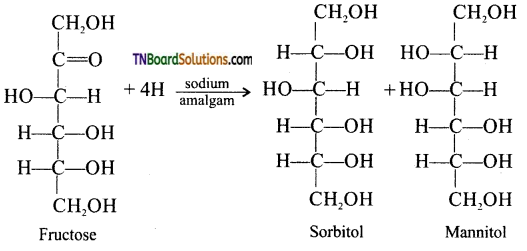
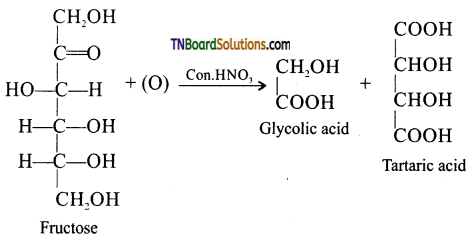
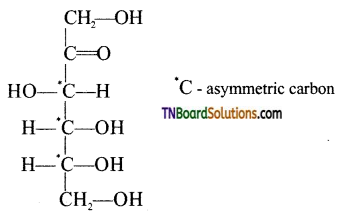

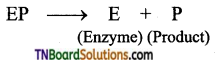
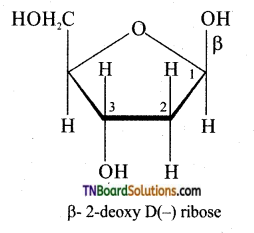
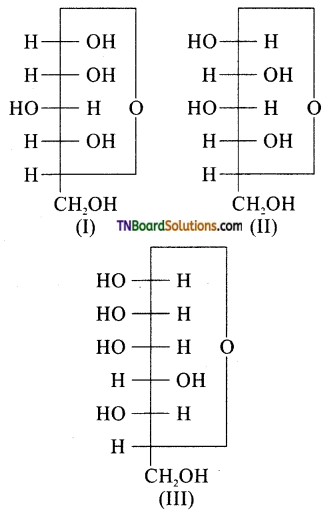
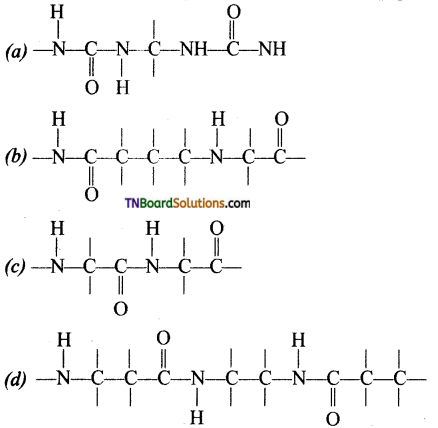
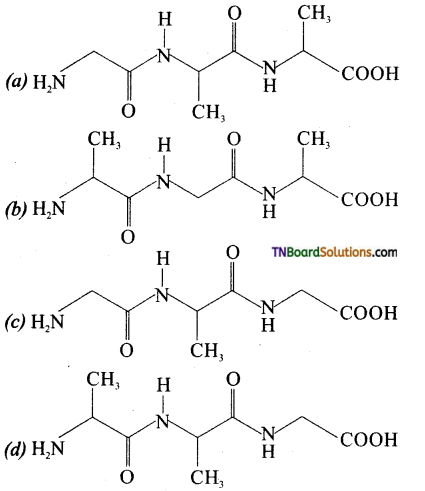

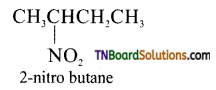
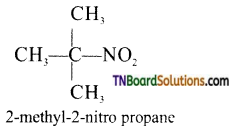

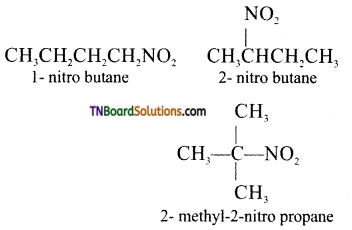

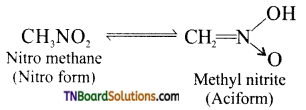
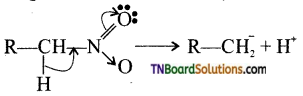

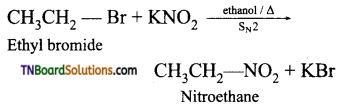
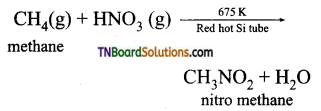
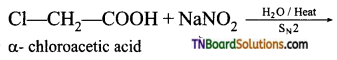
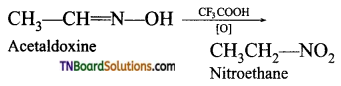

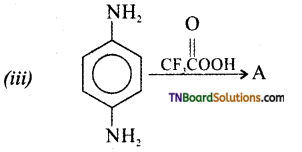
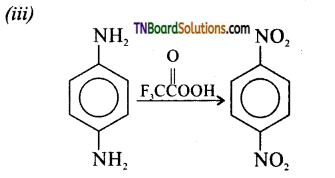
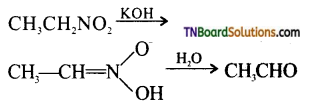
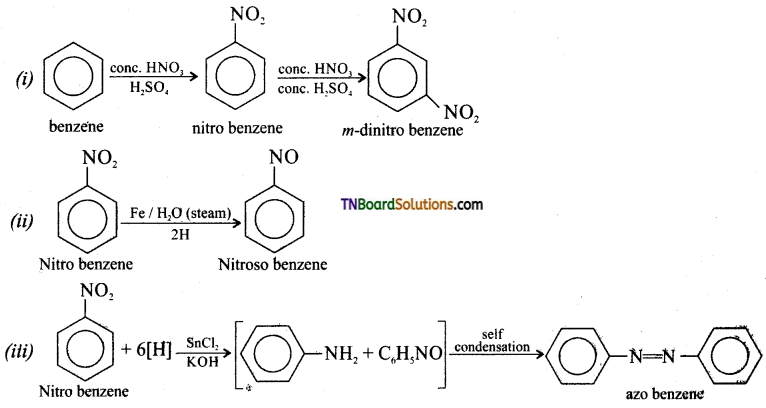
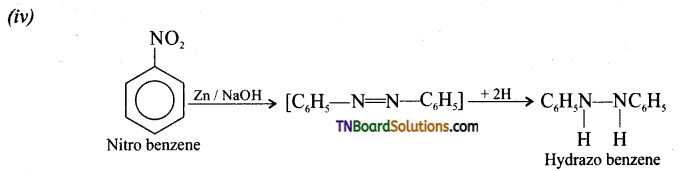

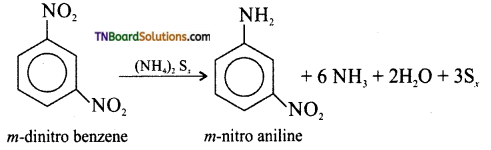
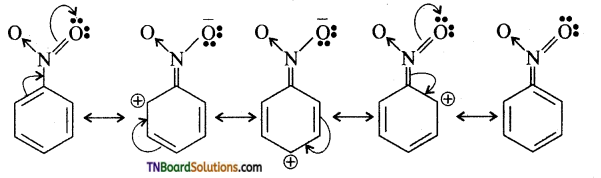
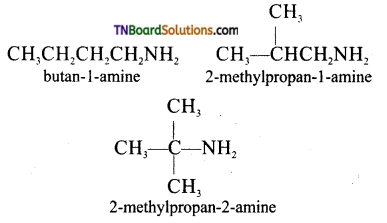
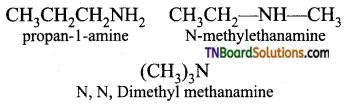
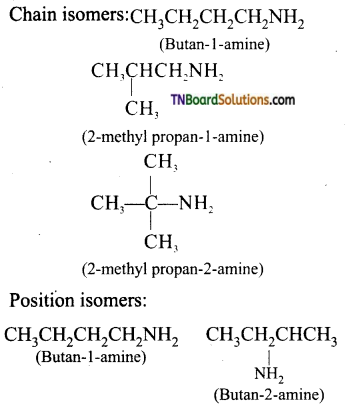
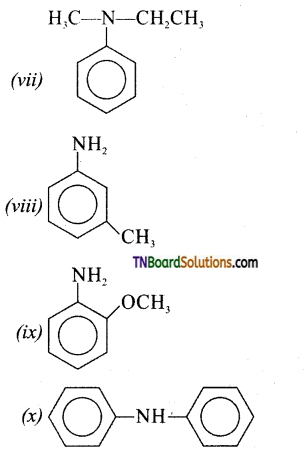
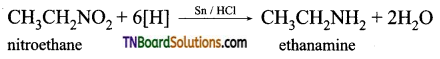
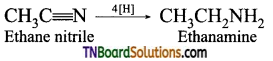

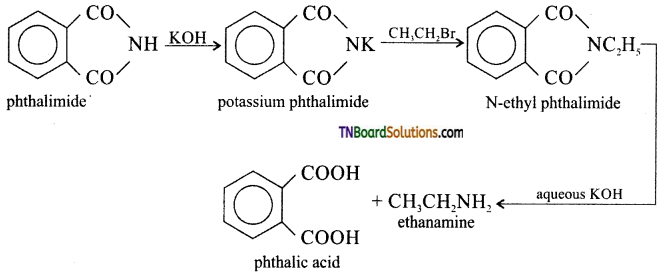
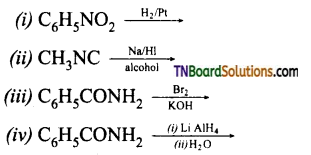

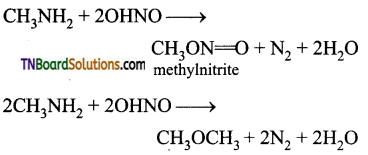
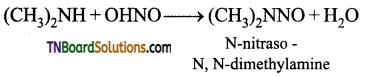
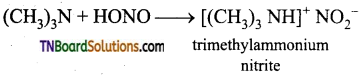
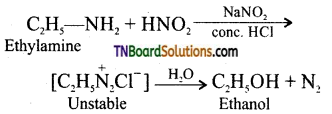


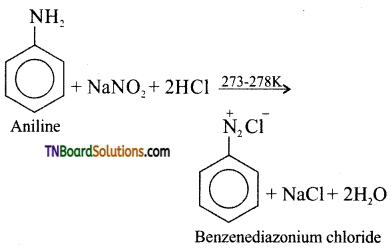
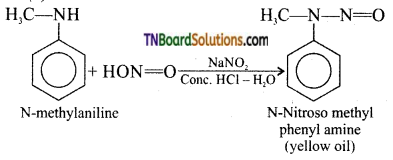
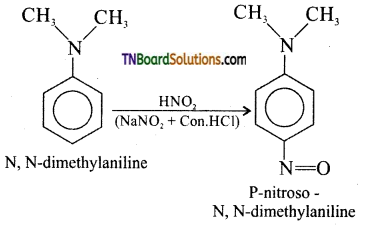
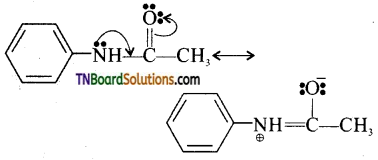
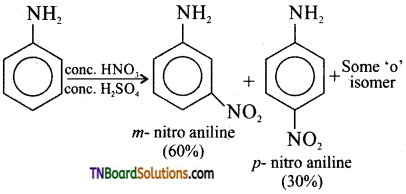
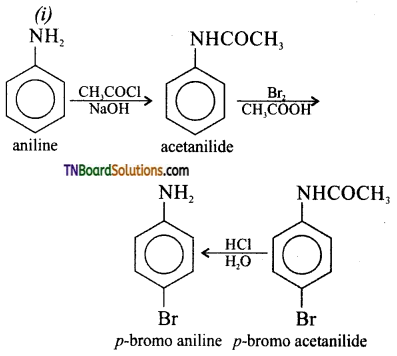
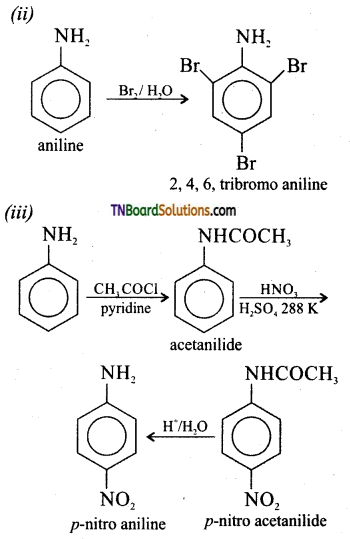
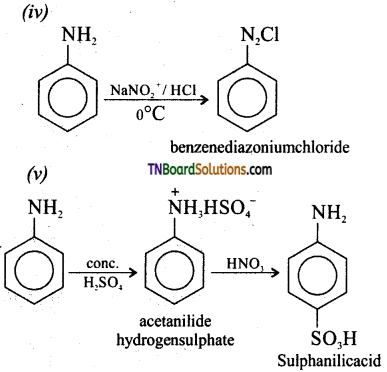

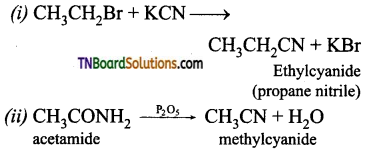
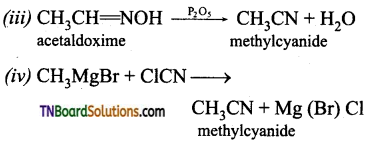
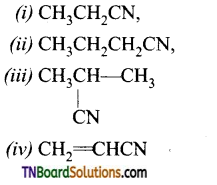
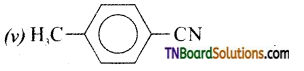
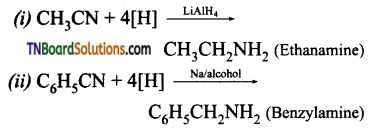

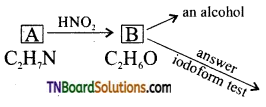
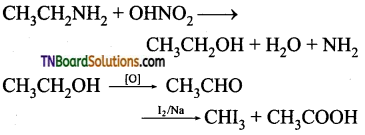
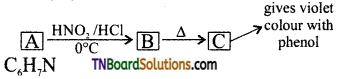
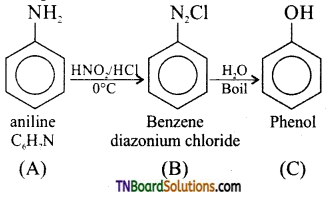

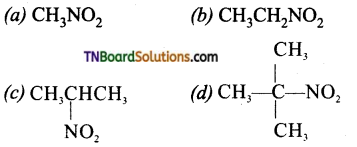
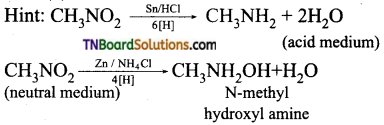

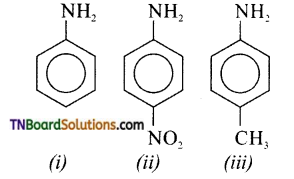
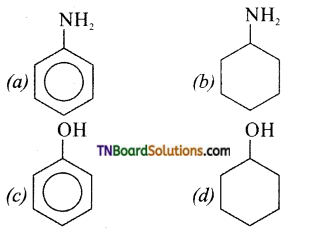
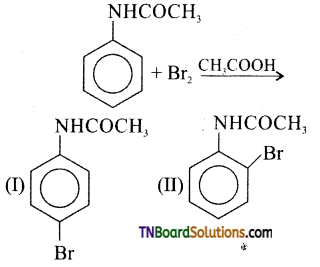
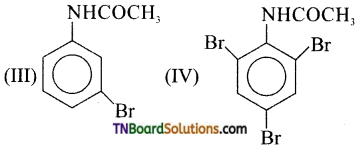
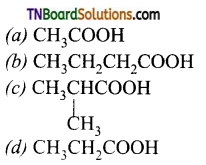
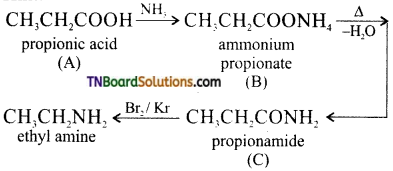
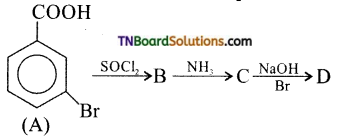
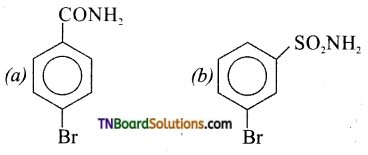
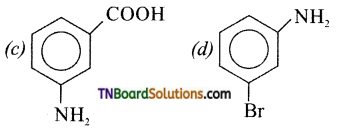
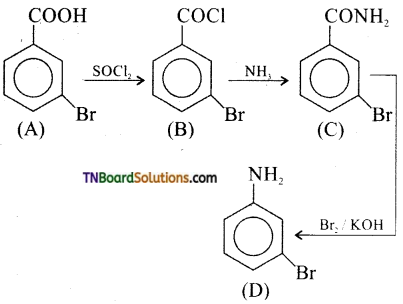
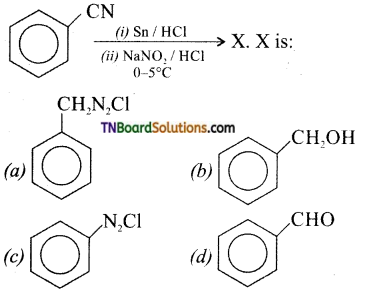
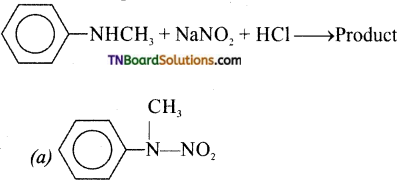
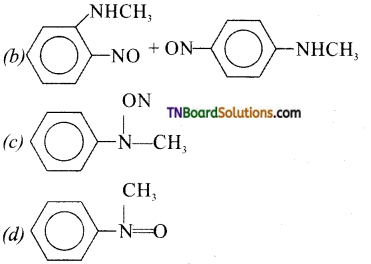
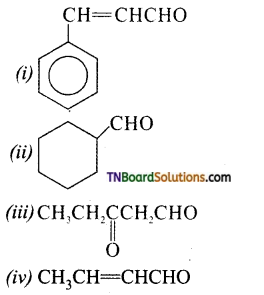
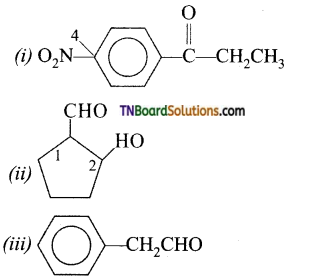
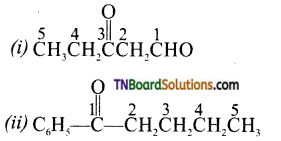
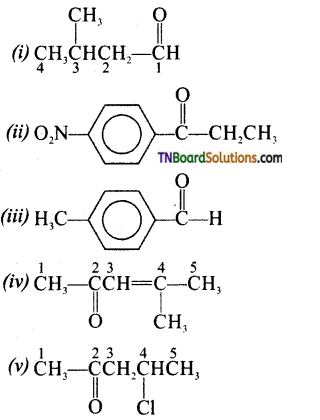
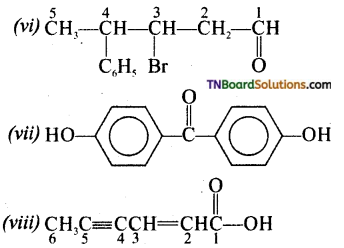

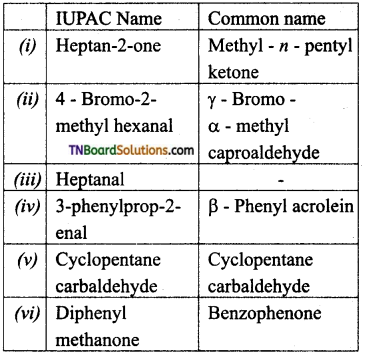
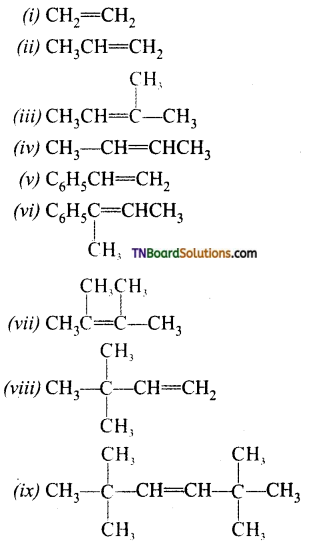
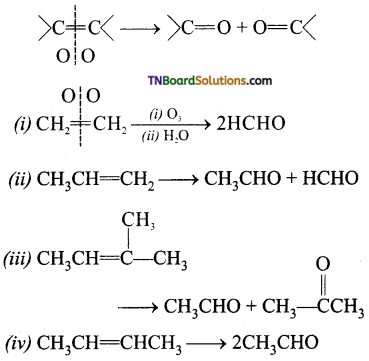
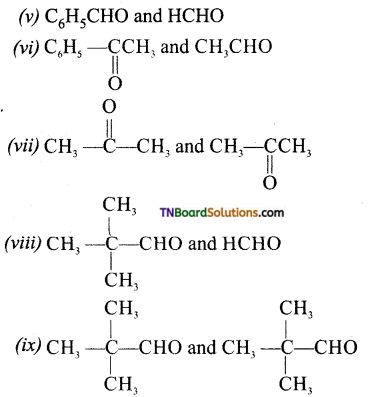
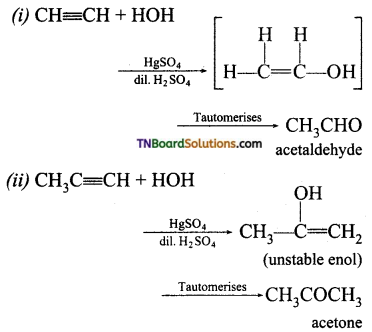
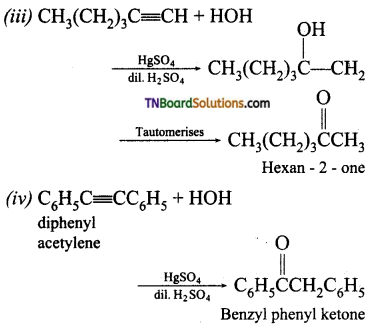
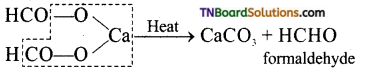
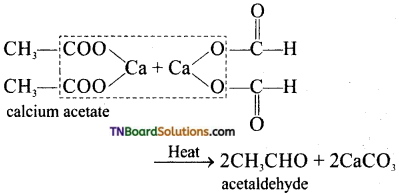
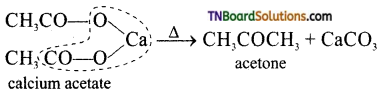
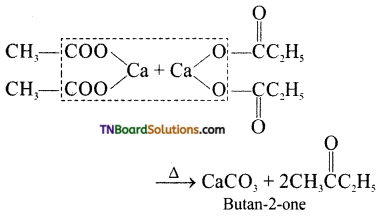
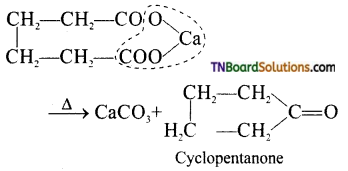
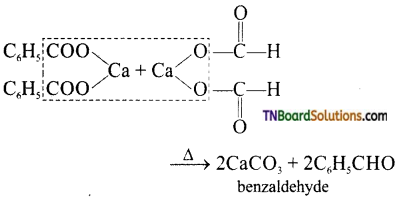

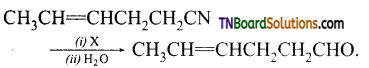
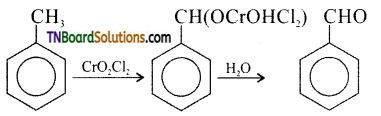
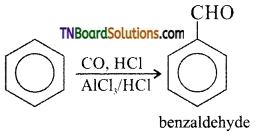
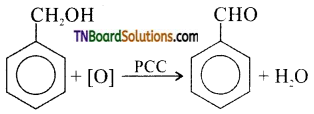
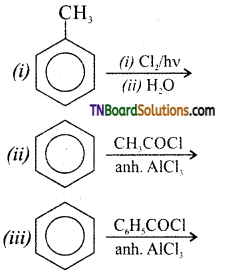
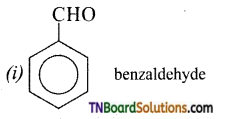
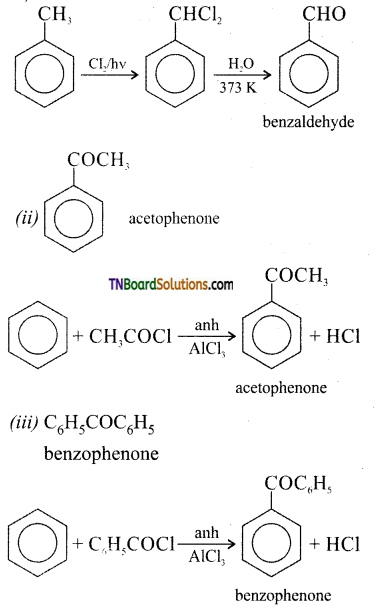
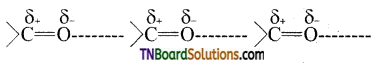
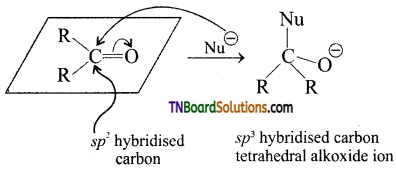
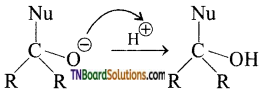
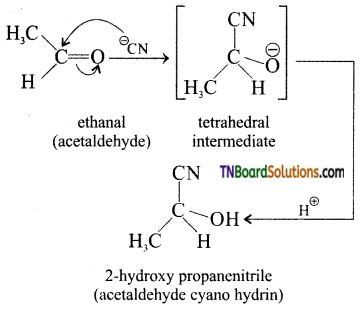


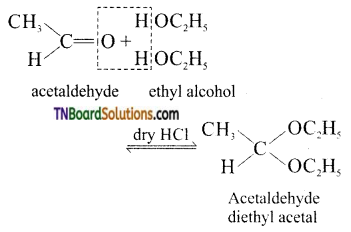
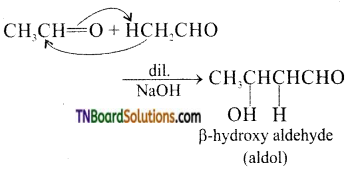
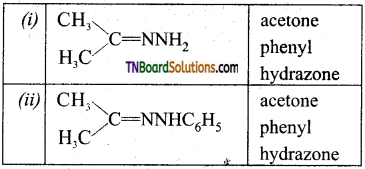

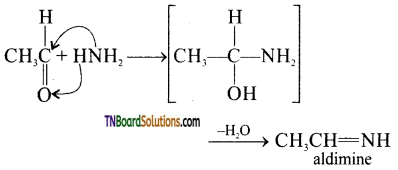
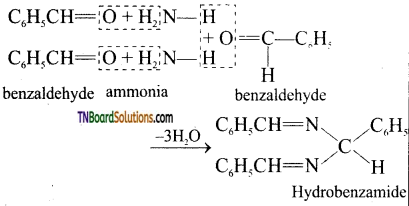
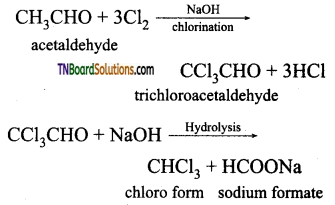
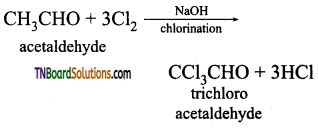
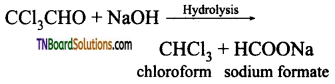
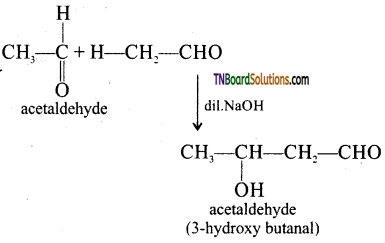

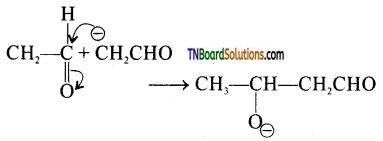
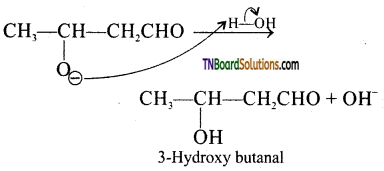
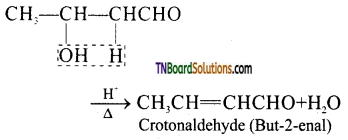
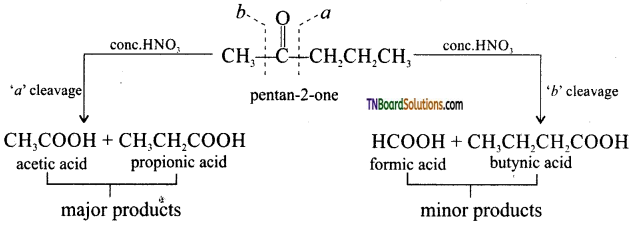
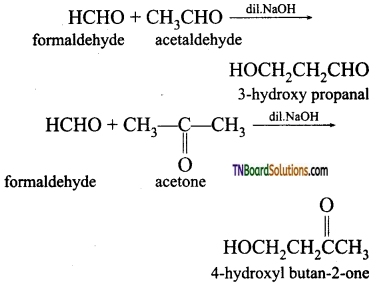
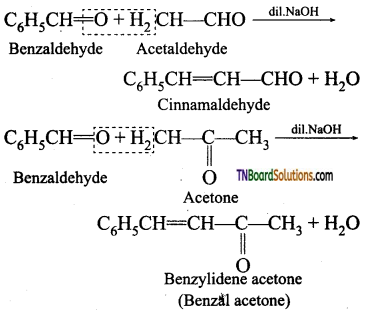
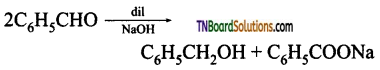
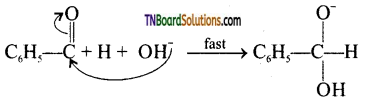
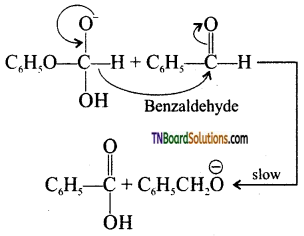
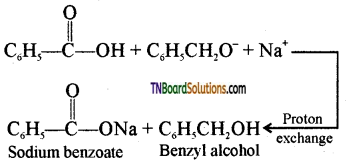
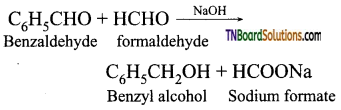
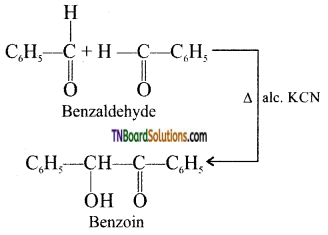
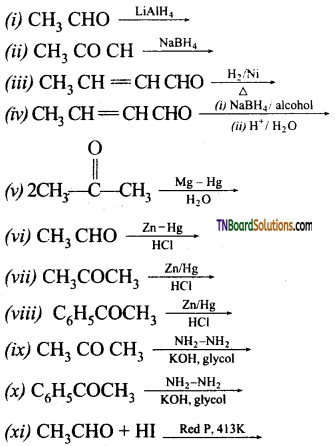
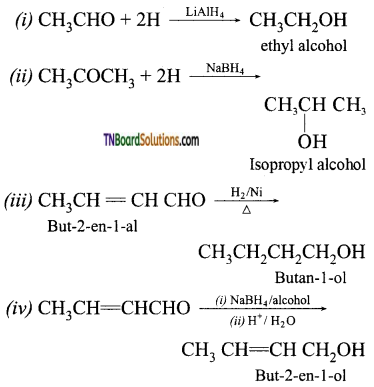
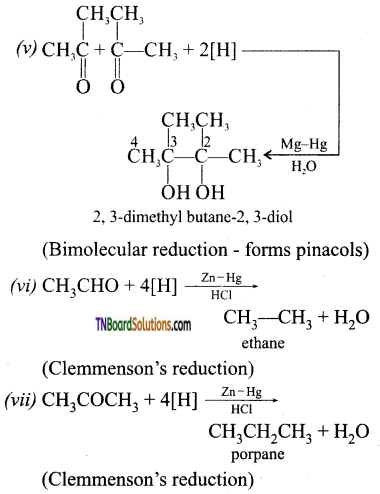
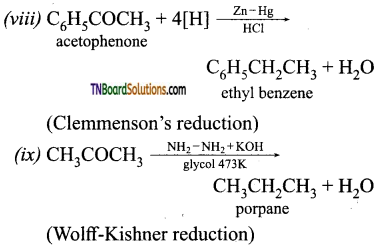
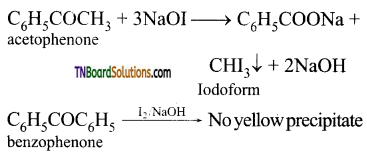

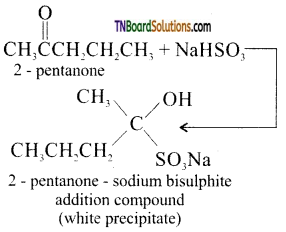

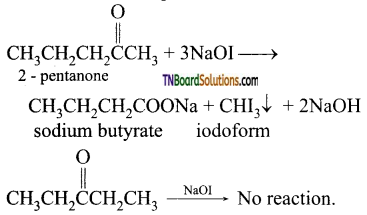
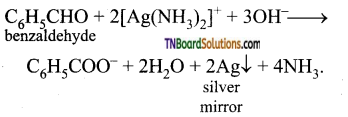
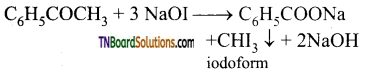
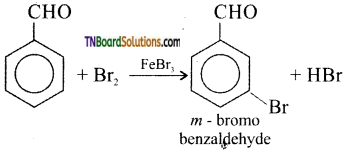
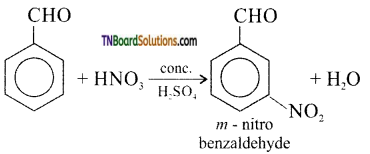
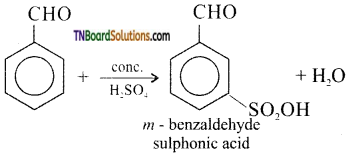
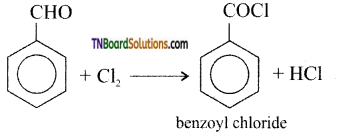
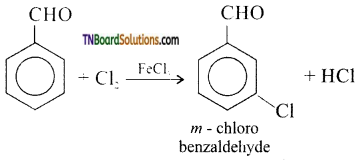
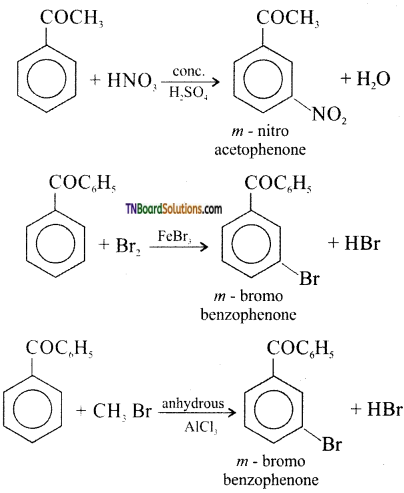


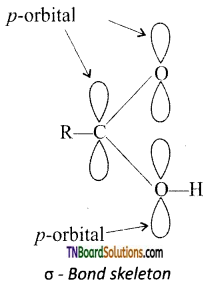
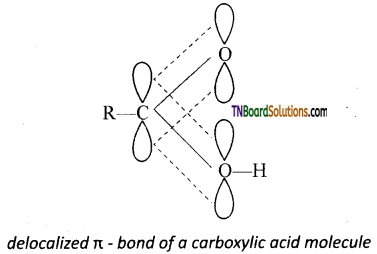
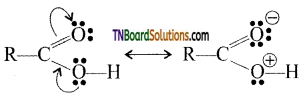
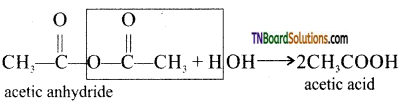
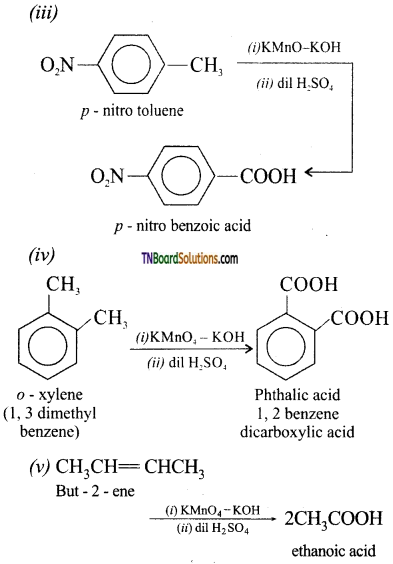
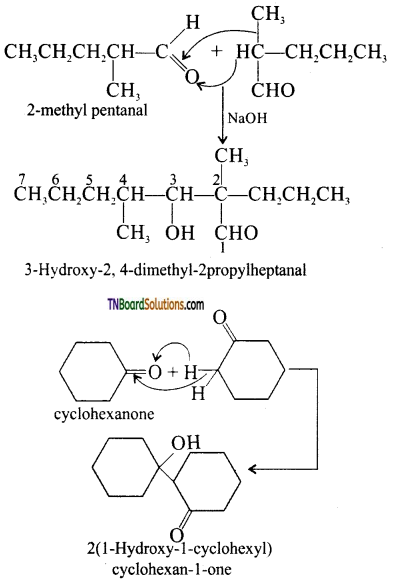
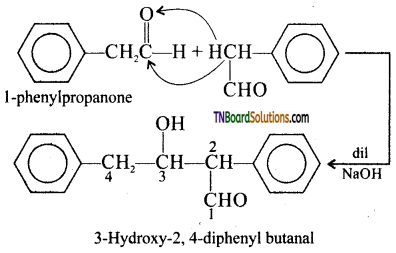
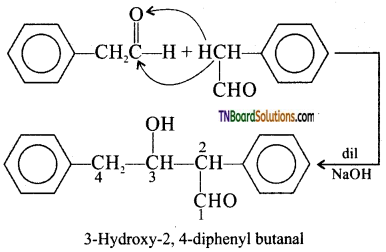
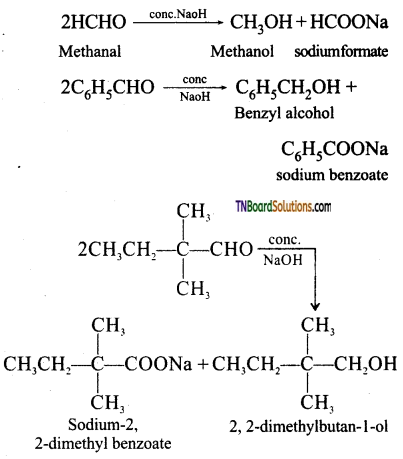
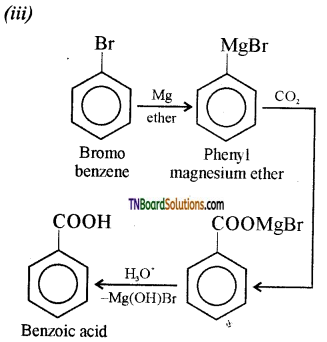

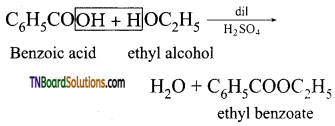

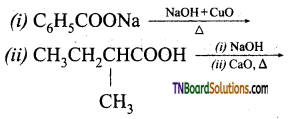
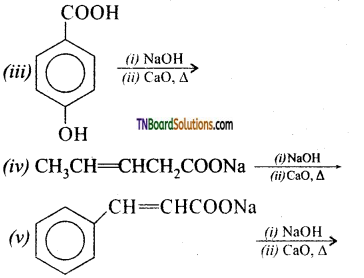
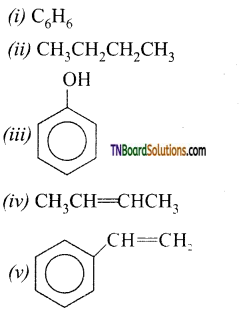
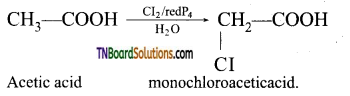
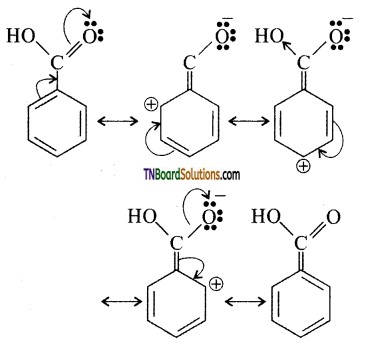
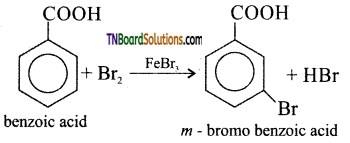
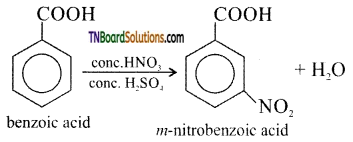
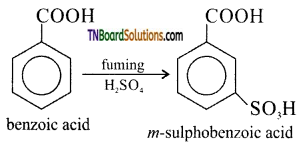
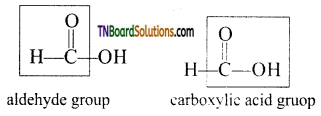
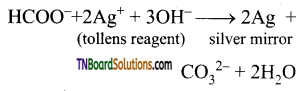
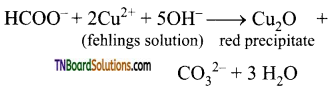
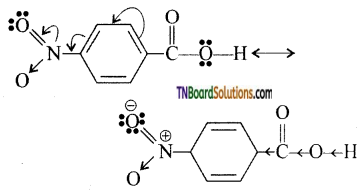
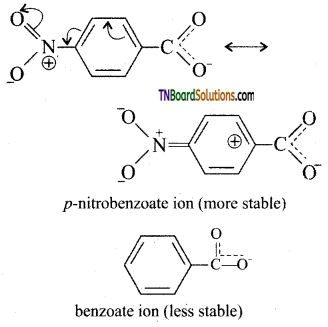
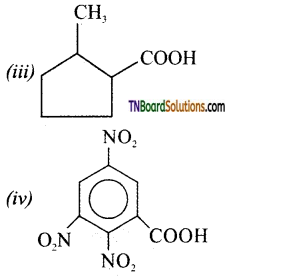
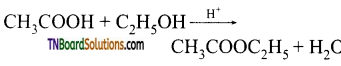
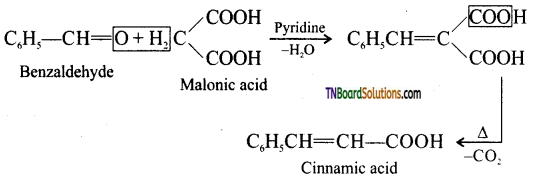
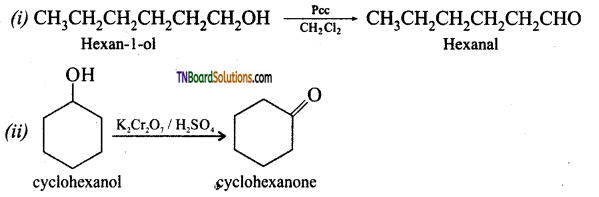
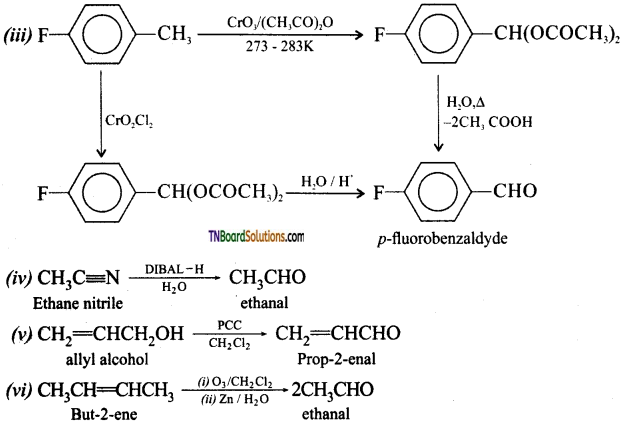
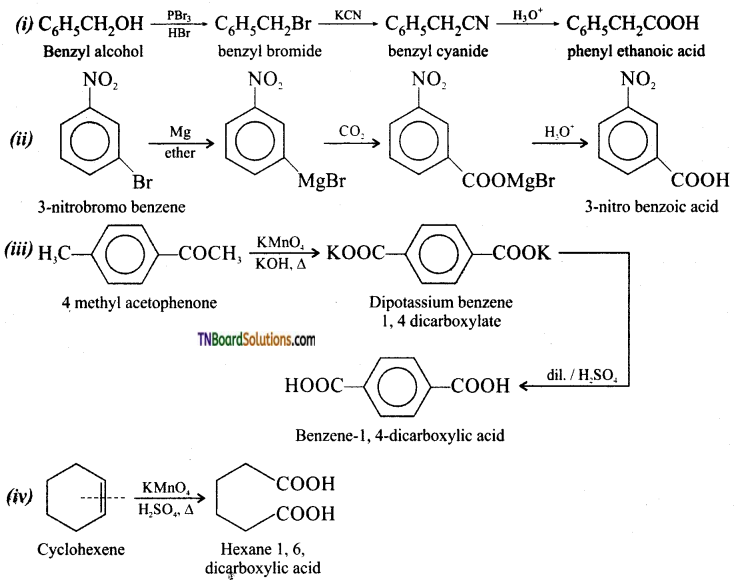
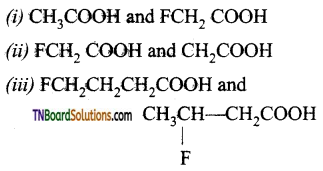
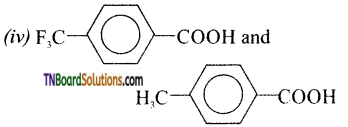
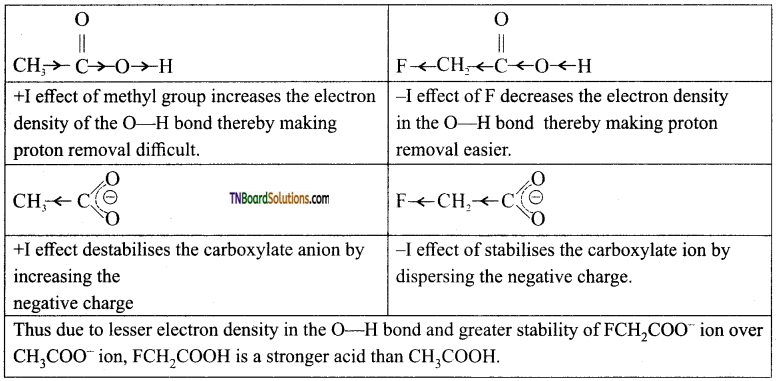
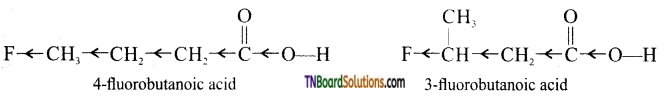
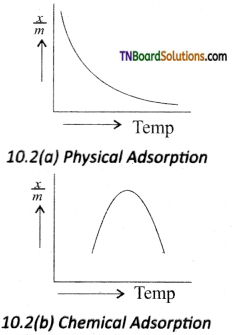
 is a stronger acid than FCH2CH2CH2COOH.
is a stronger acid than FCH2CH2CH2COOH.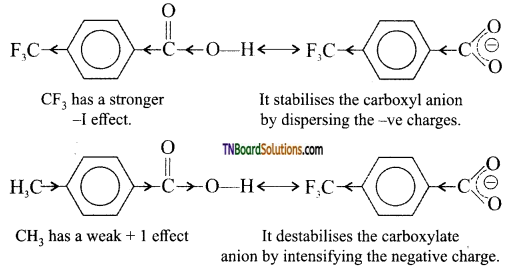
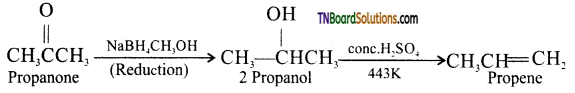
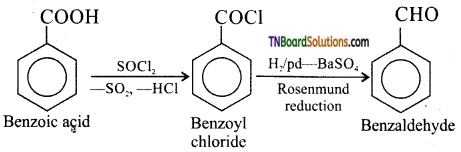

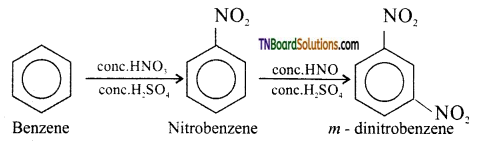
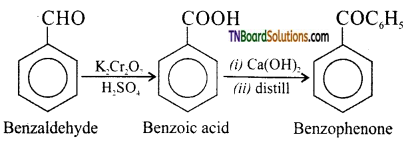
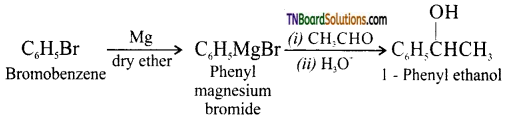
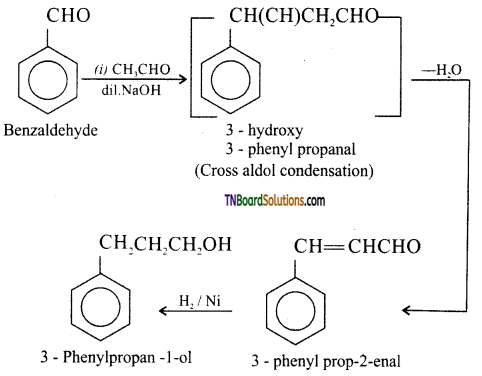
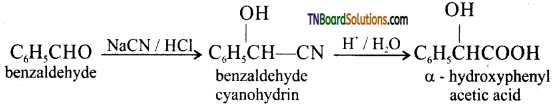
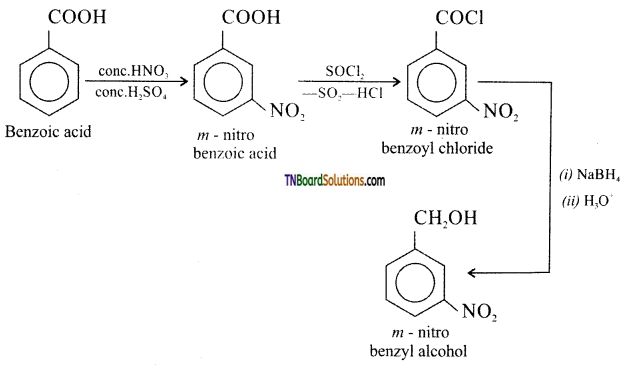

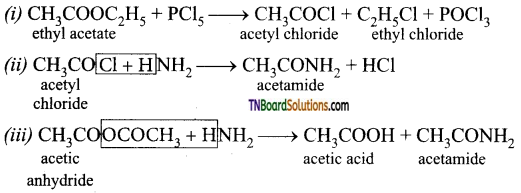
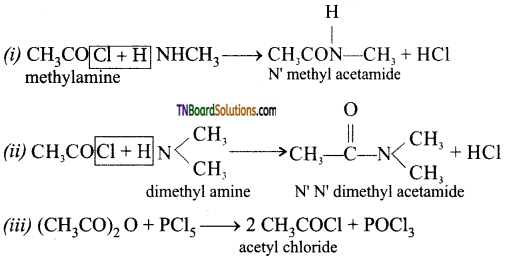
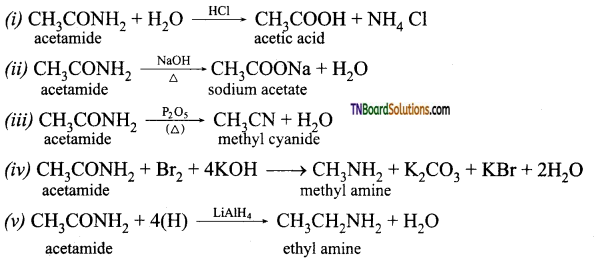
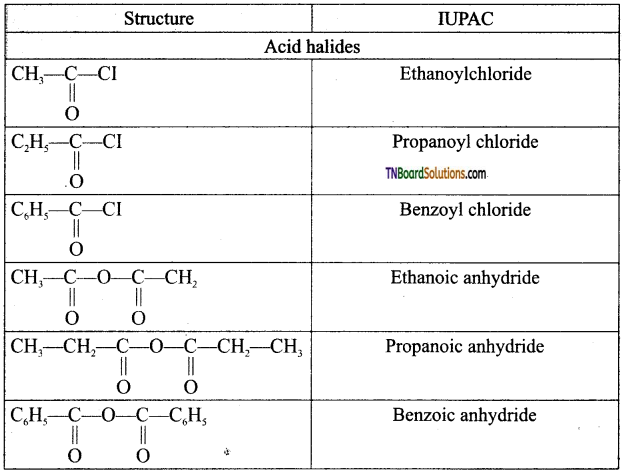
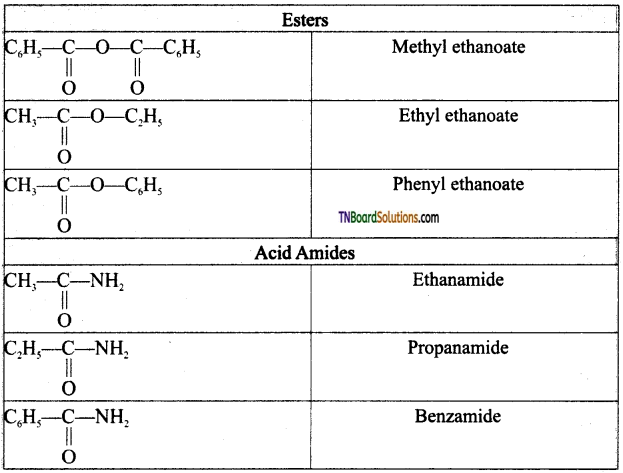


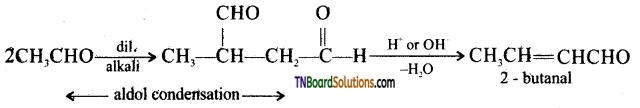
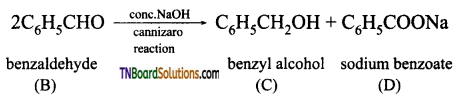

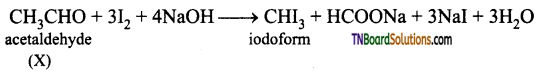
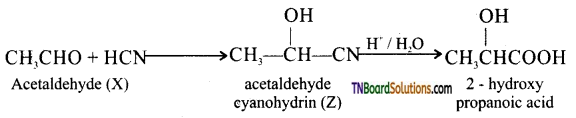
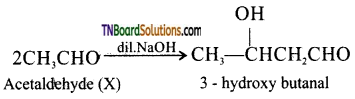
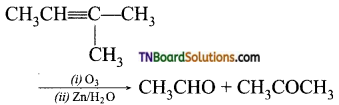
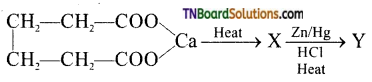
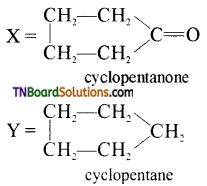
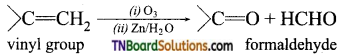
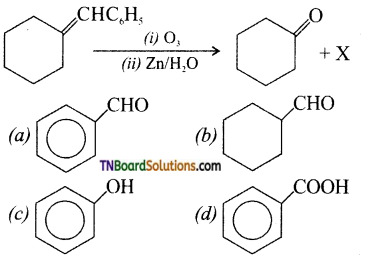
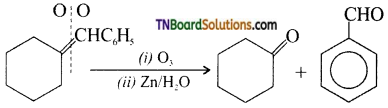
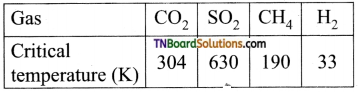
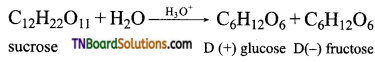
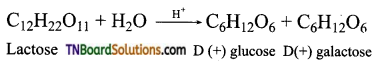
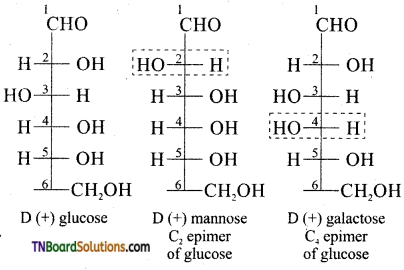
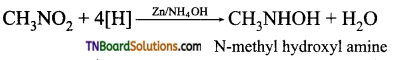
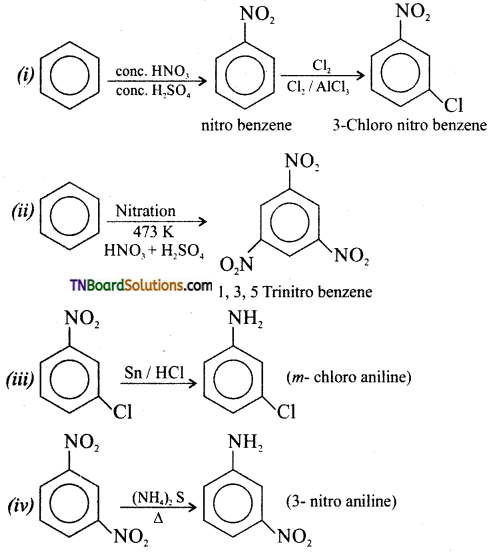
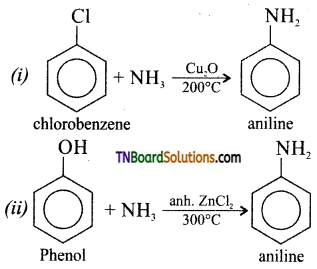
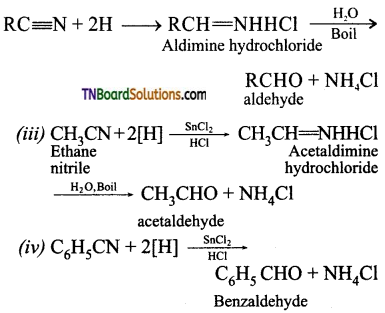
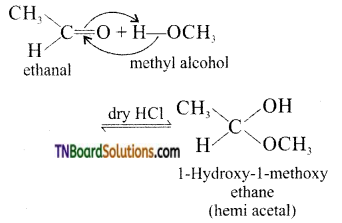
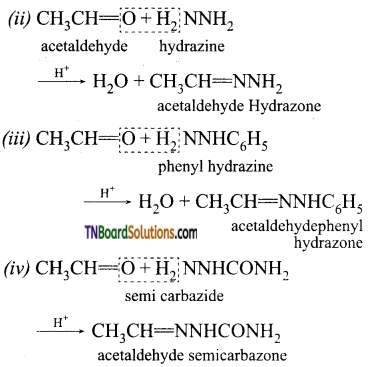
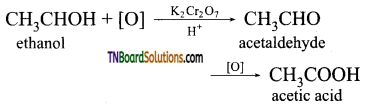
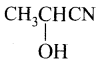 on hydrolysis gives lactic acid
on hydrolysis gives lactic acid 
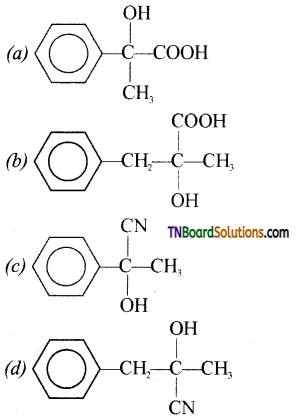
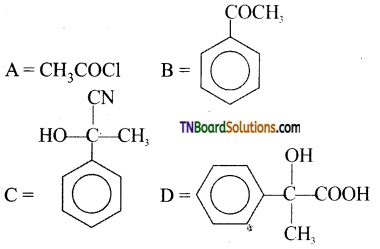
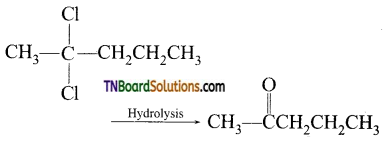
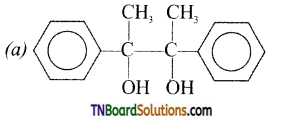
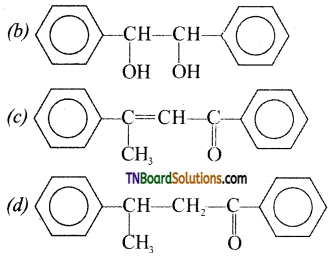
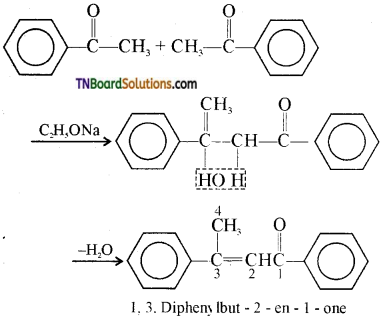
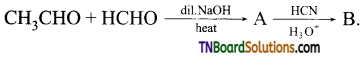
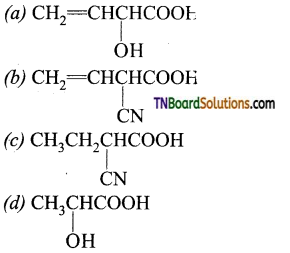
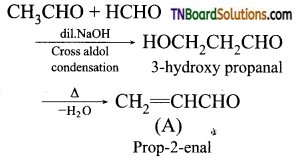
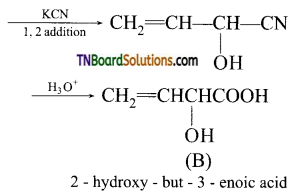
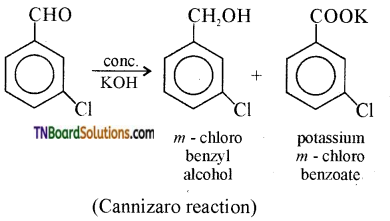
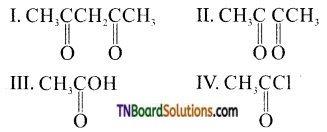
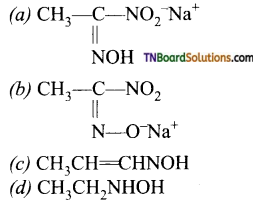
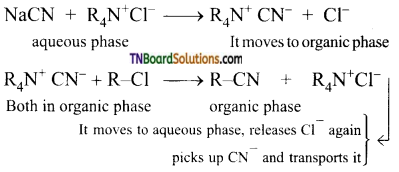
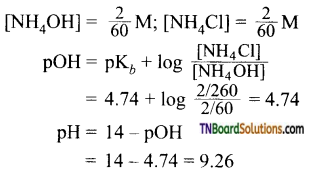
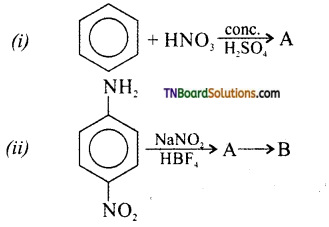
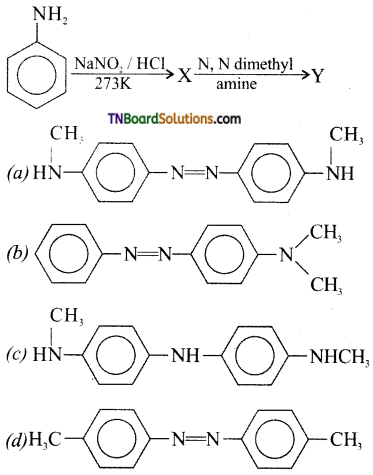
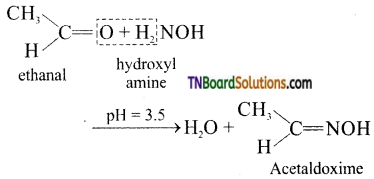
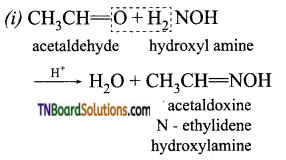
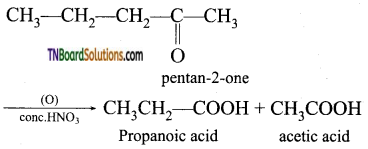
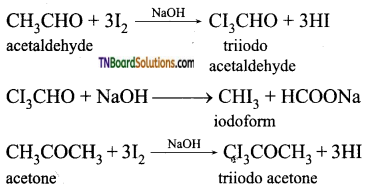
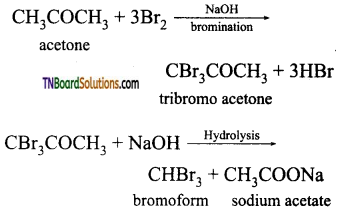
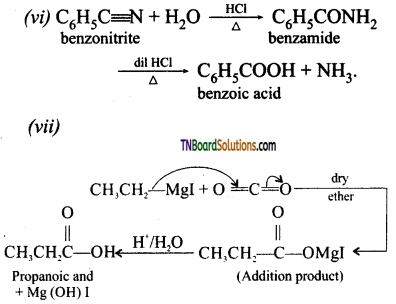
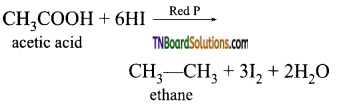
 contains a CH3CO group linked to carbon, it does not undergo iodoform test. This is because iodination occurs at the more reactive CH2 group rather than terminal CH3 which is essential for iodoform test to occur
contains a CH3CO group linked to carbon, it does not undergo iodoform test. This is because iodination occurs at the more reactive CH2 group rather than terminal CH3 which is essential for iodoform test to occur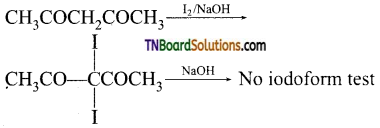
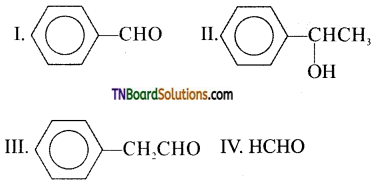
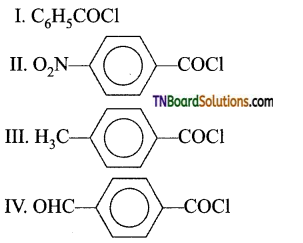
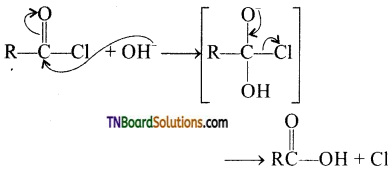
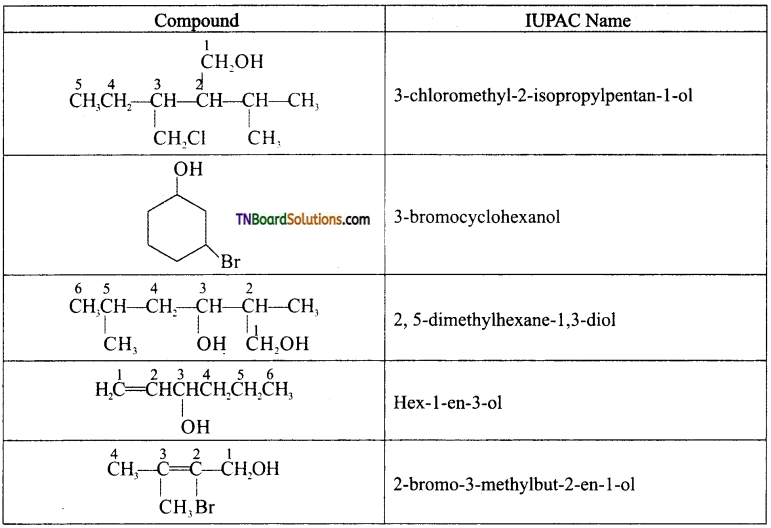
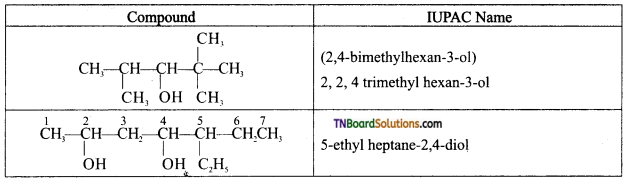
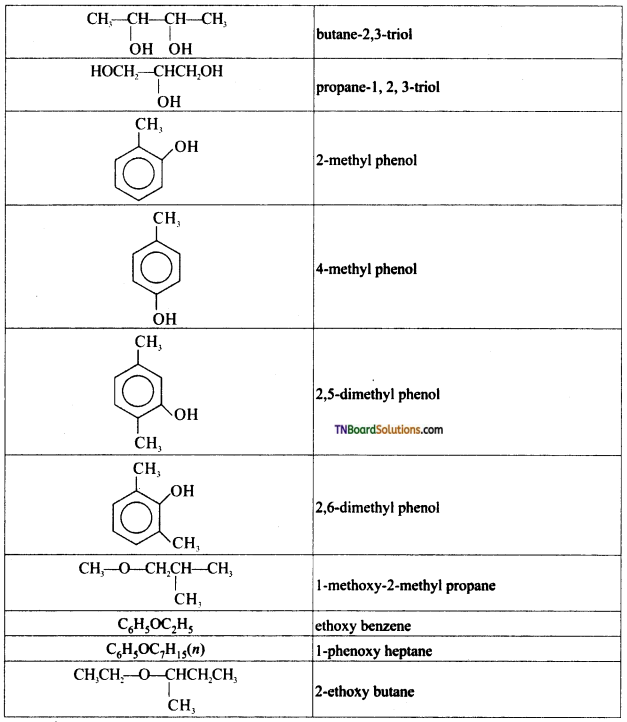
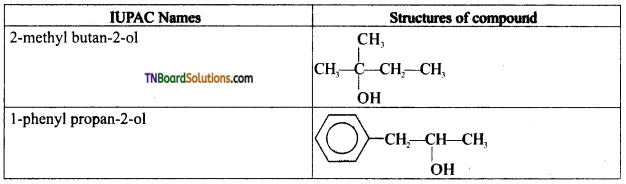

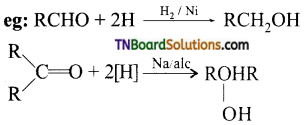
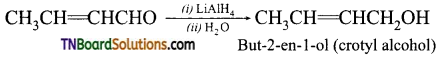
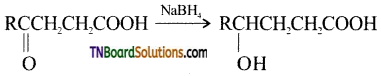
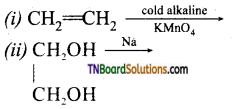
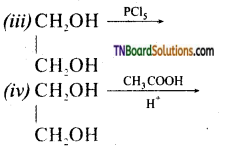
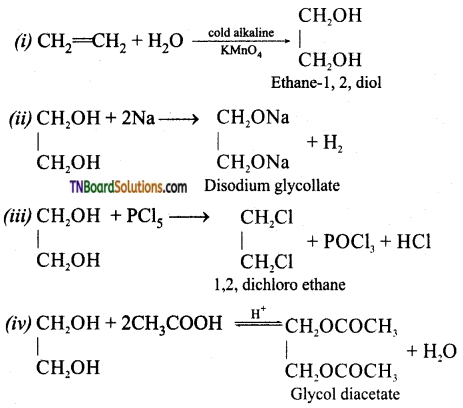
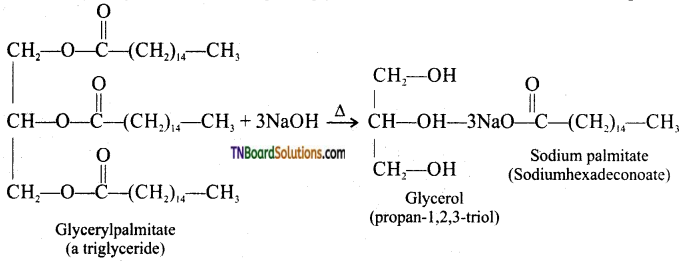
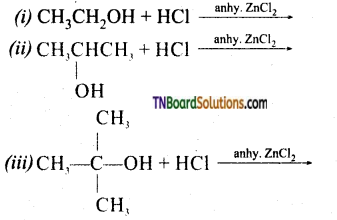

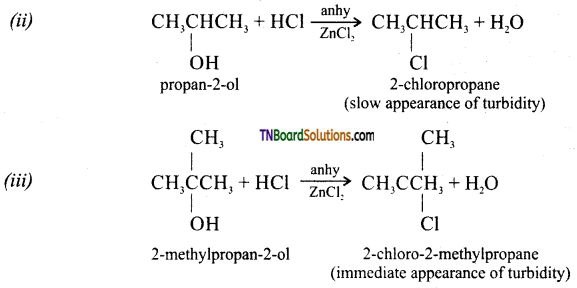
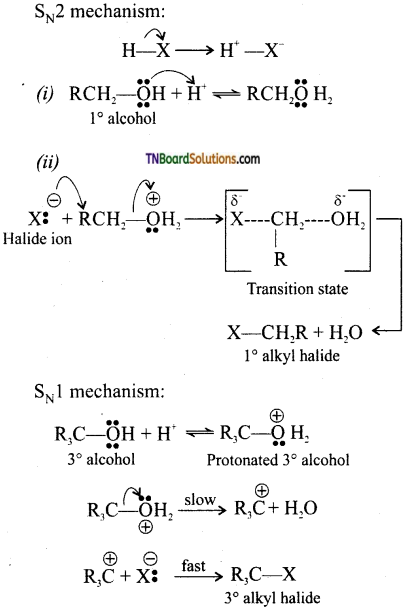
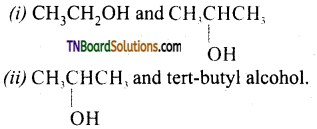
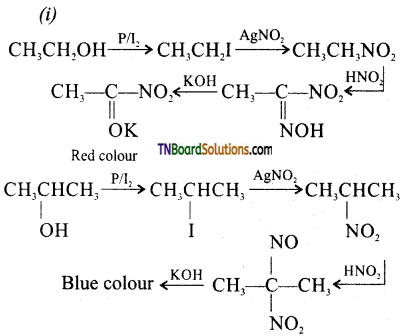
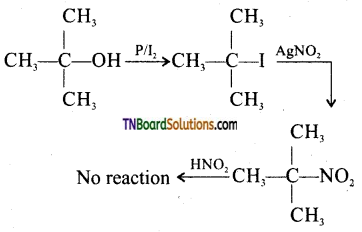
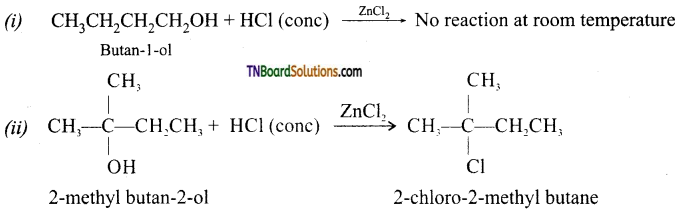
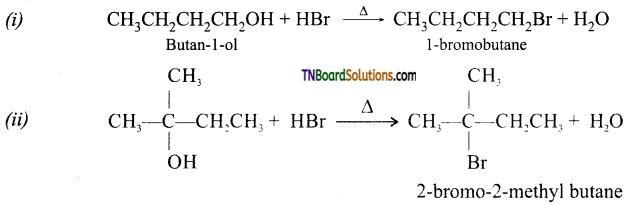
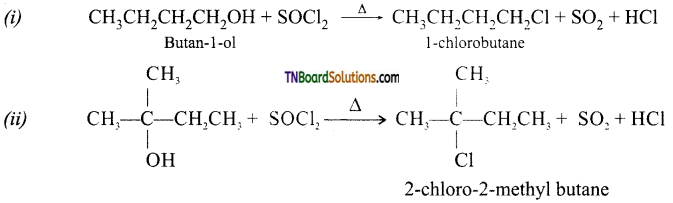
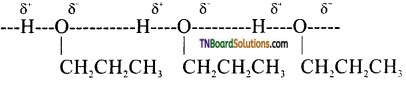

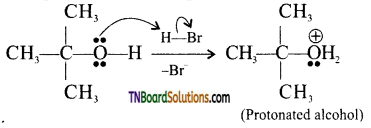
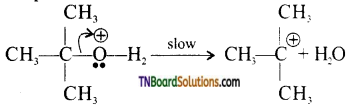
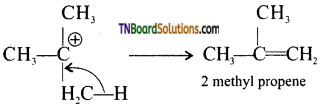
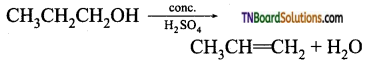


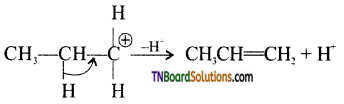
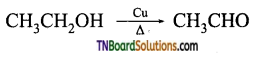
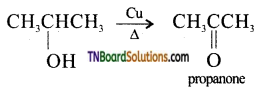
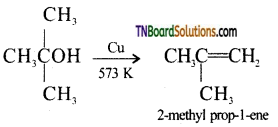
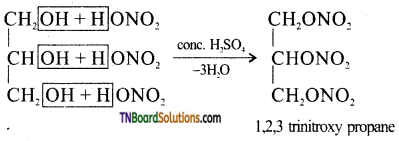
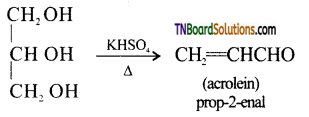
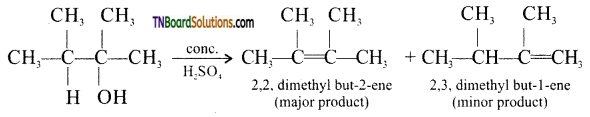
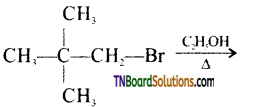
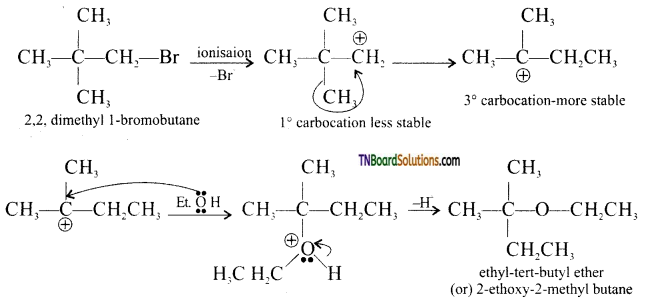
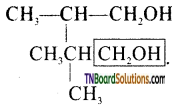
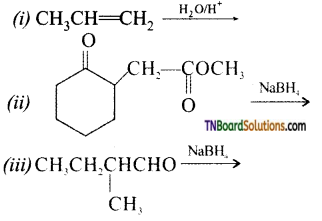
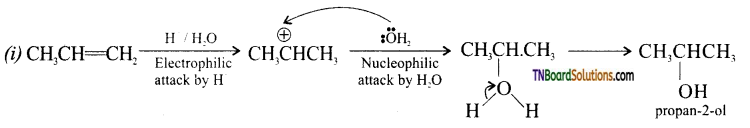
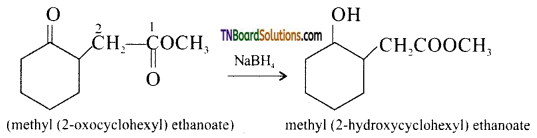
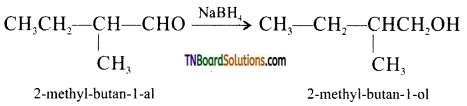
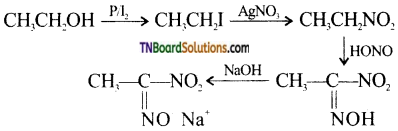
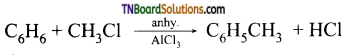
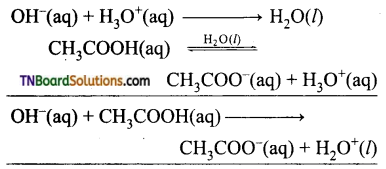
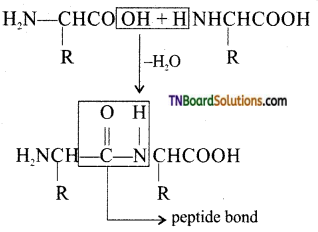
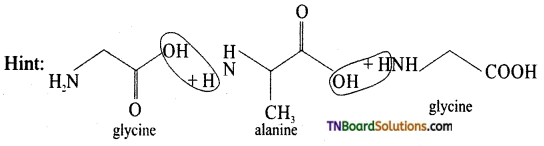
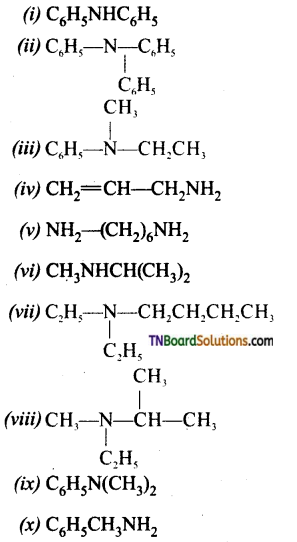
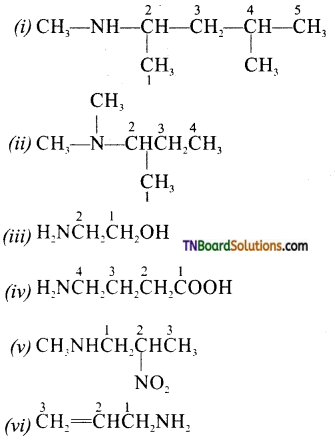
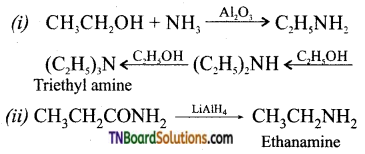
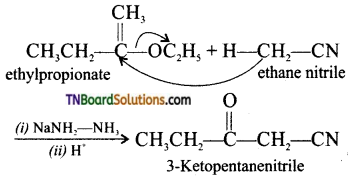
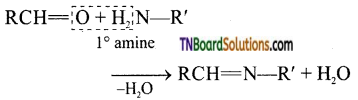
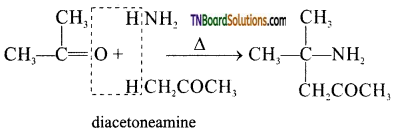
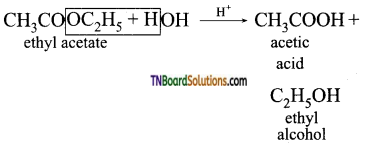
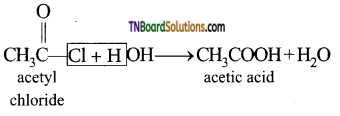
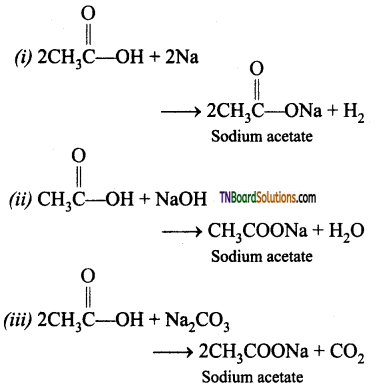
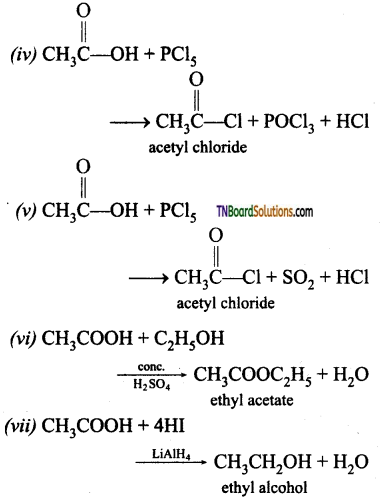
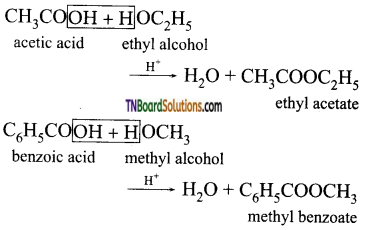
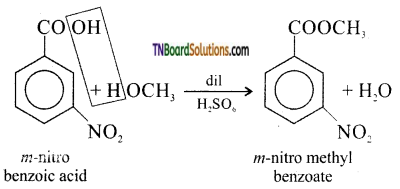
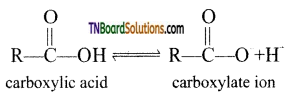
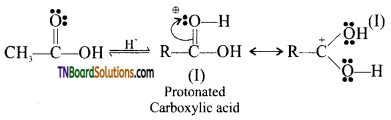
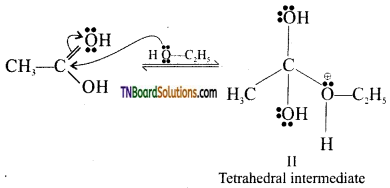
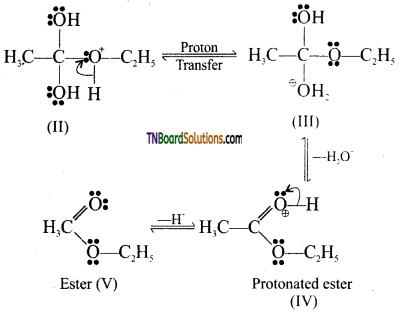
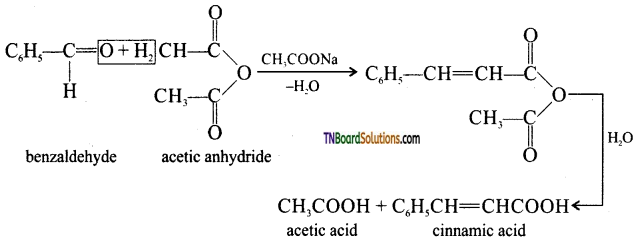
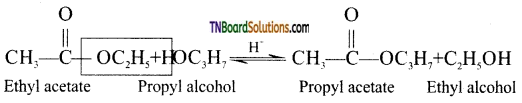
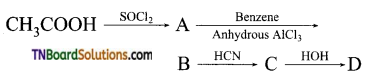
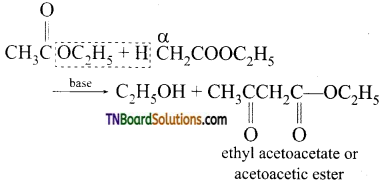
 from an alcohol and an organic acid in the presence of a catalyst is known as esterification. In the reaction, the ‘OH’ of the carboxylic acid and ‘H’ of the alcoholic group are removed as water.
from an alcohol and an organic acid in the presence of a catalyst is known as esterification. In the reaction, the ‘OH’ of the carboxylic acid and ‘H’ of the alcoholic group are removed as water.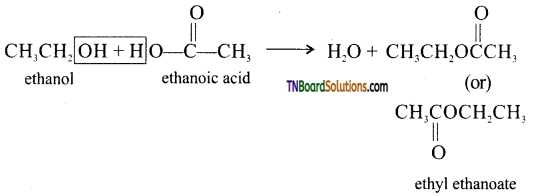
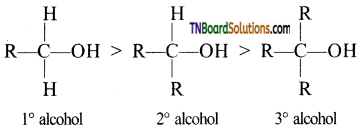
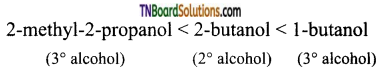
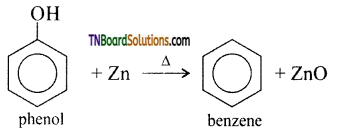
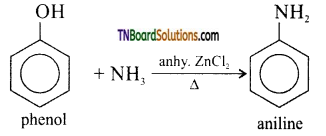
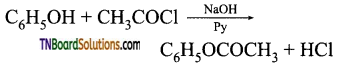
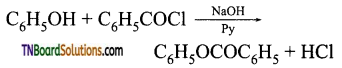
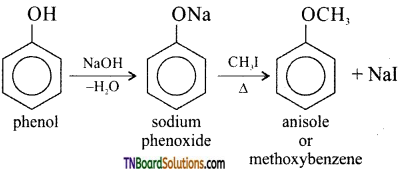
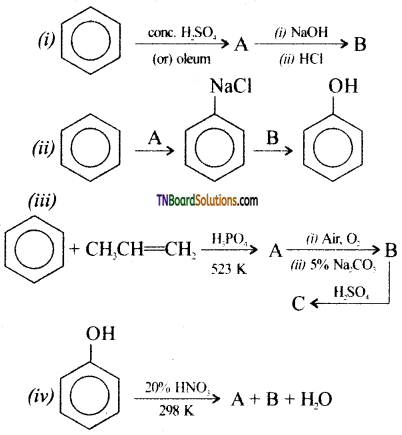
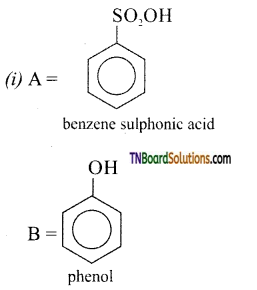
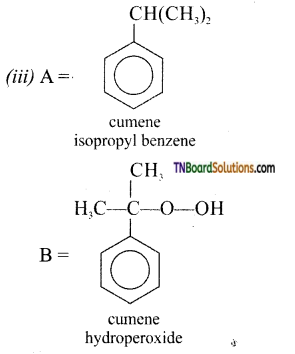
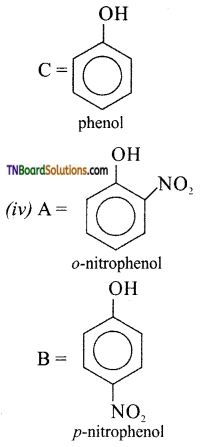
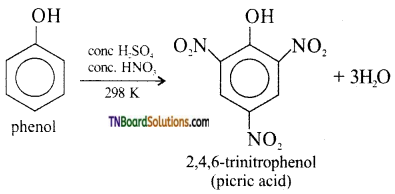
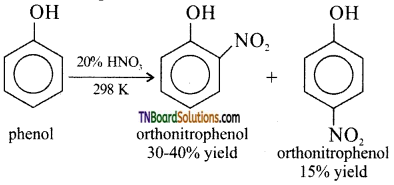
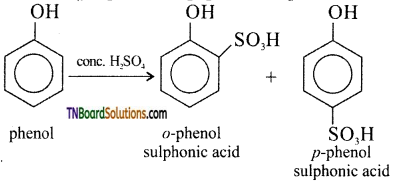
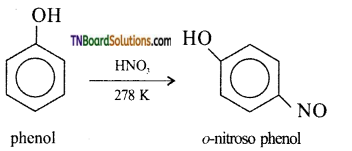
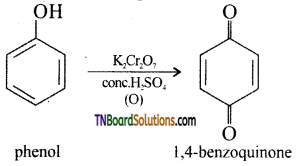
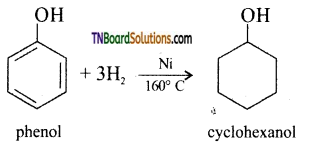
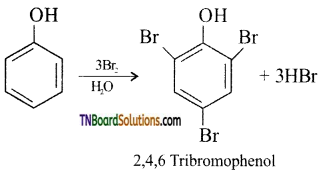
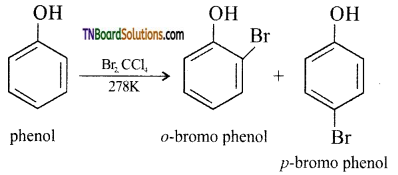
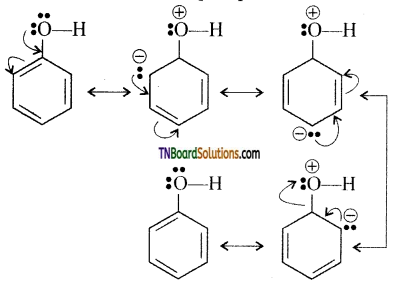
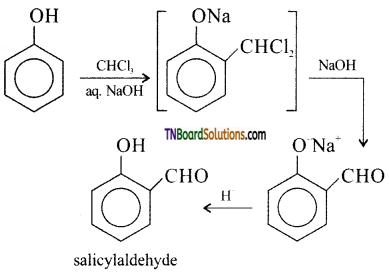
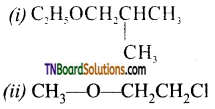
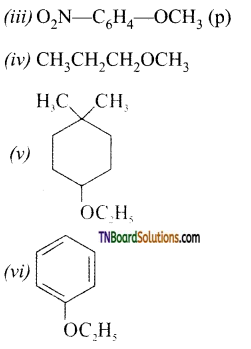
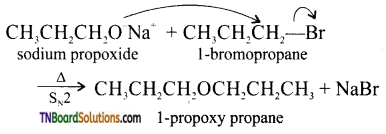
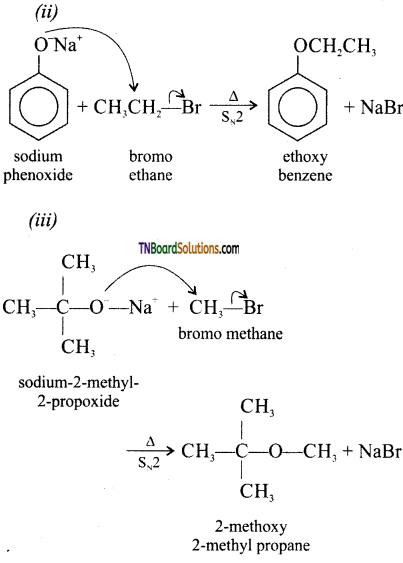
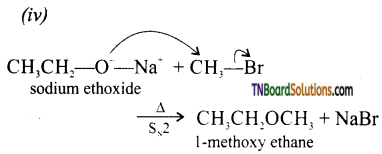
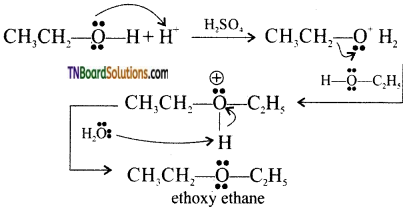
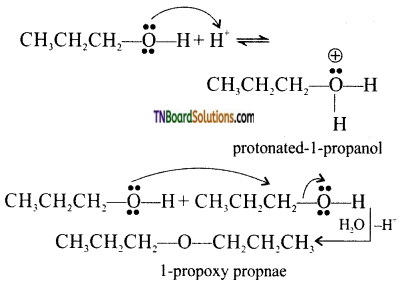
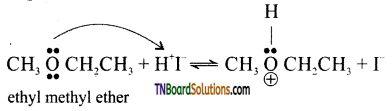
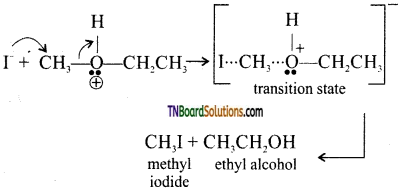
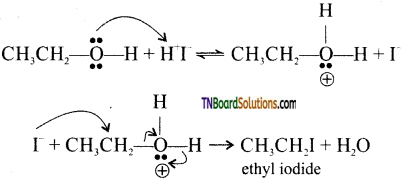
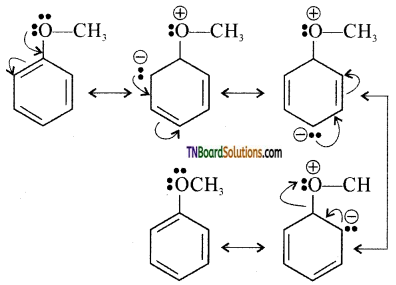


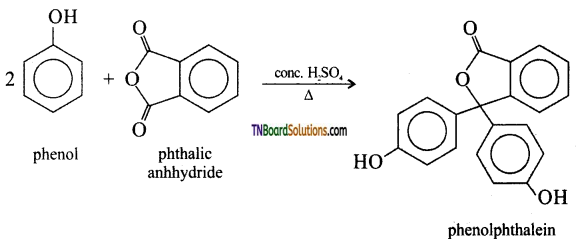
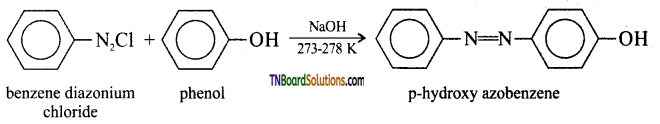


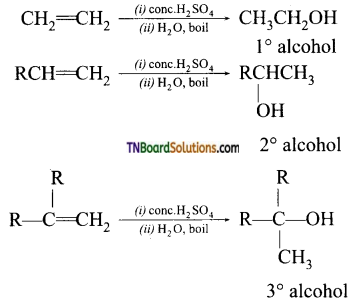
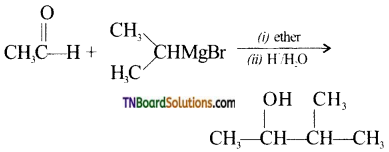
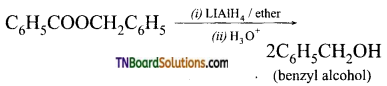
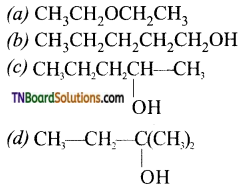
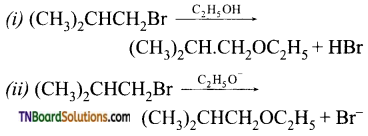

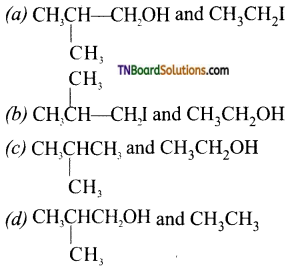
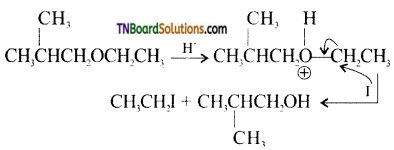
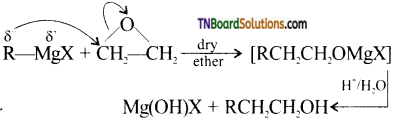
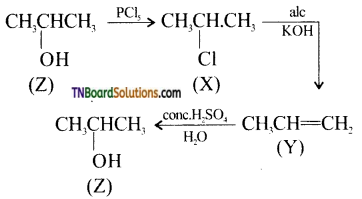
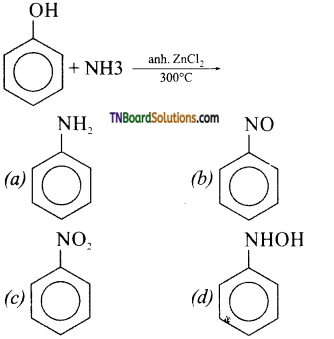
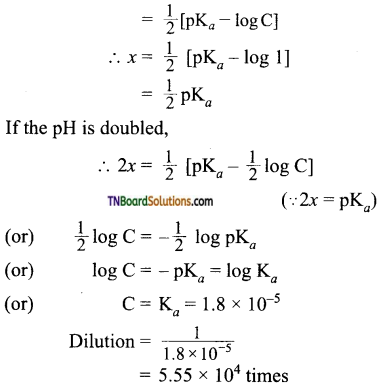
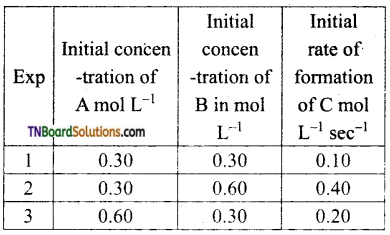
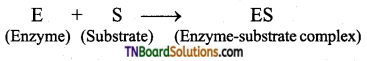
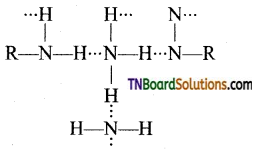
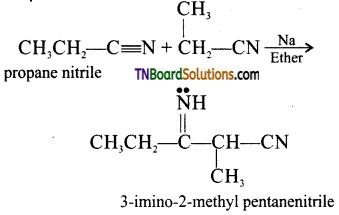
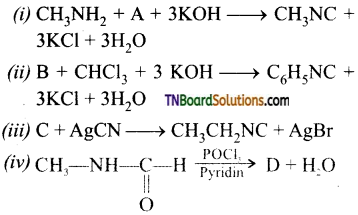
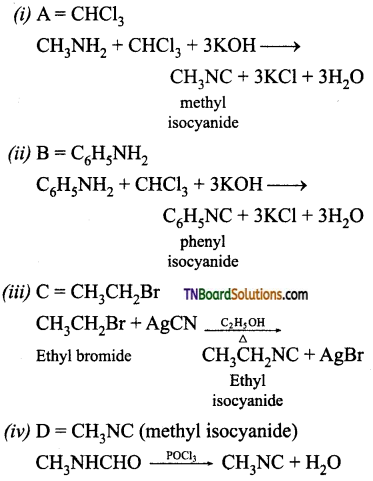
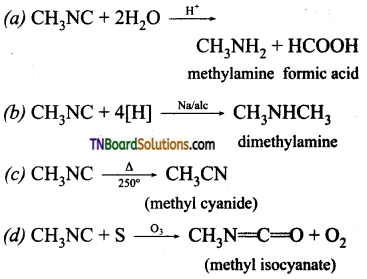
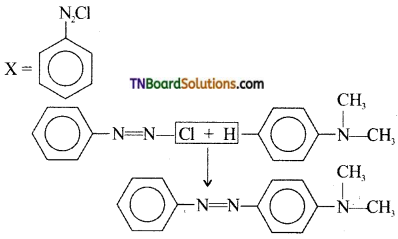
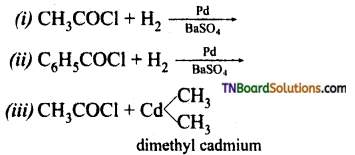
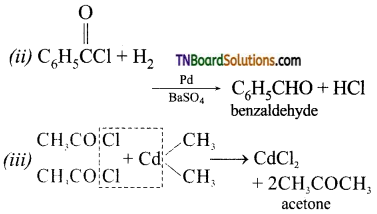
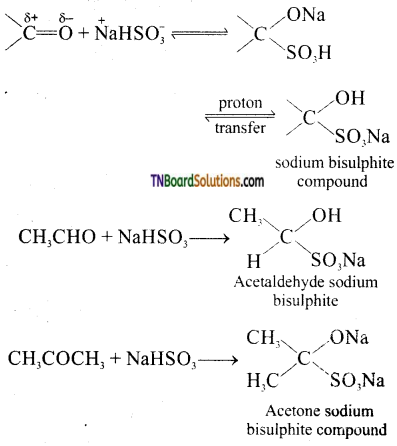
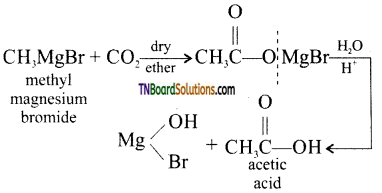
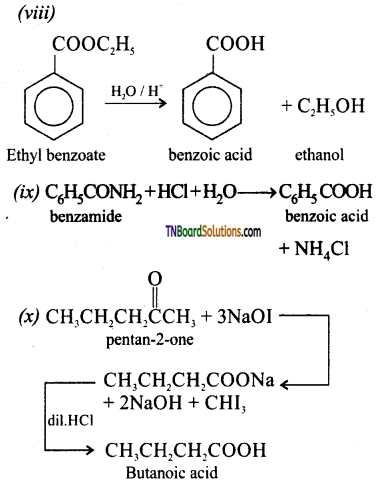
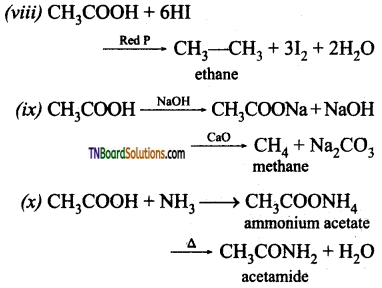
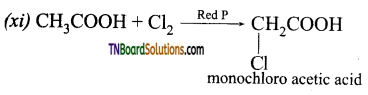
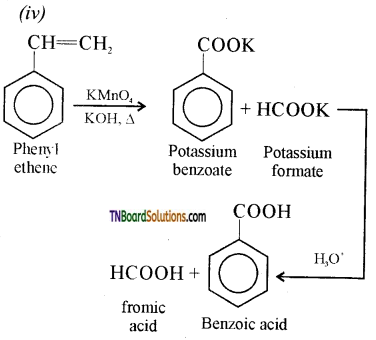
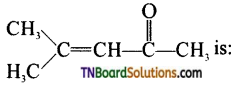
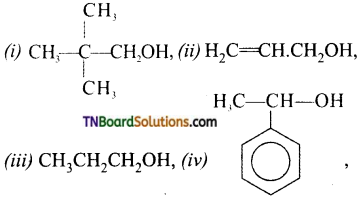
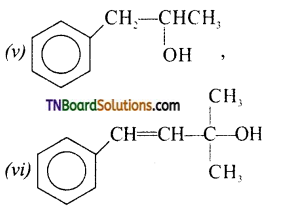
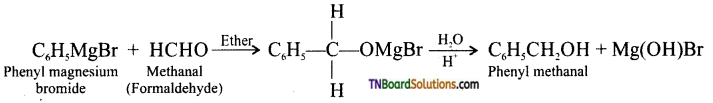
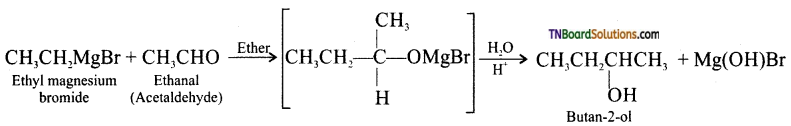
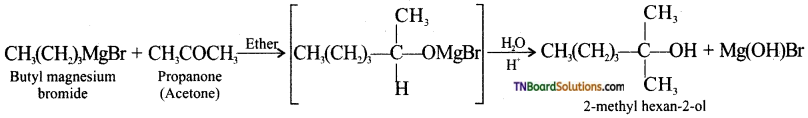
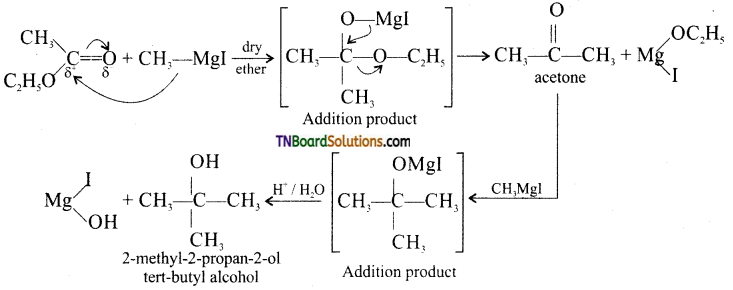
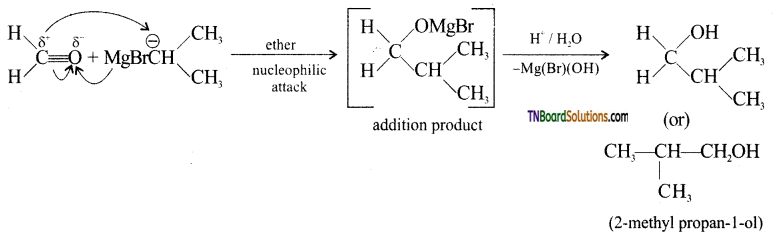
 with RMgX leads to the formation of:
with RMgX leads to the formation of: