Students get through the TN Board 12th Chemistry Important Questions Chapter 15 Chemistry in Everyday Life which is useful for their exam preparation.
TN State Board 12th Chemistry Important Questions Chapter 15 Chemistry in Everyday Life
Answer the following questions.
Question 1.
Define the term chemotherapy.
Answer:
The specific treatment of a disease by using medicine is known as chemotherapy.
Question 2.
Define therapeutic index? What is its use?
Answer:
It is defined as the ratio between the maximum tolerated dose of a drug (above which it becomes toxic) and the minimum curative dose (below which it becomes ineffective). Higher the values of therapeutic index safer is the drug.
![]()
Question 3.
Explain the term, target molecules or drug targets as used in medicinal chemistry.
Answer:
Drugs interact with macromolecules like proteins, carbohydrates, lipids, nucleic acids. Hence they are called target drugs. Proteins perform several roles in the body. Enzymes are crucial in communication systems and are called receptors.
Question 4.
Give examples of drugs that are grouped based on the biological effect that they produce on the recipient.
Answer:
- Antibiotic drugs: Amoxycillin, ampicillin, cefixime, cefpodoxime, erythromycin, tetracycline, etc.
- Antihypertensive drugs: propranolol, atenolol, metoprolol succinate, amlodipine, etc.
Question 5.
Streptomycin and erythromycin are classified in the same group. Justify the statement.
Answer:
Both inhibit the protein synthesis (target process) in bacteria and are classified in the same group. However, the mode of action is different. Streptomycin inhibits the initiation of protein synthesis while erythromycin prevents the incorporation of new amino acids to the protein. These drugs are grouped based on the biological system/process that targets the recipient.
Question 6.
Write a short note on enzymes as drug targets.
Answer:
In all living systems, the biochemical reactions are catalysed by enzymes. Hence, these enzyme actions are highly essential for the normal functioning of the system. If their normal enzyme activity is inhibited, then the system will be affected. This principle is usually applied to kill many pathogens.
In enzyme catalysed reactions, the substrate molecule binds to the active site of the enzyme by means of the weak interaction such as hydrogen bonding, Van der waals force etc., between the amino acids present in the active site and the substrate.
When a drug molecule that has a similar geometry (shape)as the substrate is administered, it can also bind to the enzyme and inhibit its activity. In other words, the drug acts as an inhibitor to the enzyme catalyst. These types of inhibitors are often called competitive inhibitors.
In certain enzymes, the inhibitor molecule binds to a different binding site, which is commonly referred to as an allosteric site and causes a change in its active site geometry (shape). As a result, the substrate cannot bind to the enzyme. These types of inhibitors are called allosteric inhibitors.
![]()
Question 7.
How do drugs interact with targets? (or) Give a brief account of drug-target interaction.
Answer:
Drugs interact with macromolecules like carbohydrates, proteins, nucleic acids lipids present in the cell. The macromolecules perform various functions in the body, eg: Proteins perform several roles in the body.
- Proteins that act as biological catalysts are called enzymes.
- Proteins that are crucial to a communication system in the body are called receptors.
- Proteins that carry polar molecules across the membranes are called carrier proteins.
In their catalytic activity enzymes perform two major functions.
- The first function of an enzyme is to hold the substrate molecule for a chemical reaction.
- The second function of the enzyme is to provide functional groups which will attack the substrate to carry out the chemical reaction.
Question 8.
How do drugs interact with enzymes? (or) Give a brief account of drug-enzyme interaction.
Answer:
Drugs that inhibit any two of the activities mentioned in Q7 are called enzyme inhibitors. Enzyme inhibitors can block the binding site thereby preventing the binding of the substrate to the active site and hence inhibit the catalytic activity of enzymes.
Drugs inhibit the attachment of natural substrate on active site in two ways:
- Drugs that compete with the natural substrate for their attachment on the active sites of enzymes are called competitive inhibitors.
- Same drugs, however, do not bind on the active site but bind to different sites of the enzyme which is called the allosteric site.
This binding of the drug (inhibitor) at the allosteric site changes the shape of the active site of the enzyme in such a way that the natural substrate cannot recognize it. Such enzymes are called noncompetitive inhibitors.
If the bond between an enzyme and the drug (inhibitor) is a strong covalent bond that cannot be broken easily then the enzyme is blocked permanently. The body then degrades the enzyme-drug (inhibitor) complex and synthesis the new enzyme.
Question 9.
Explain the terms (i) Competitive inhibitors, (ii) Allosteric inhibitors.
Answer:
(i) Competitive inhibitors:
In enzyme-catalyzed reactions, the substrate molecule binds to the active site of the enzyme by means of the weak interaction such as hydrogen bonding, Van der Waals force etc., between the amino acids present in the active site and the substrate. When a drug molecule that has a similar geometry (shape)as the substrate is administered, it can also bind to the enzyme and inhibit its activity. In other words, the drug acts as an inhibitor to the enzyme catalyst. These types of inhibitors are often called competitive inhibitors.
(ii) Allosteric inhibitors:
In certain enzymes, the inhibitor molecule binds to a different binding site, which is commonly referred to as an allosteric site and causes a change in its active site geometry (shape). As a result, the substrate cannot bind to the enzyme. These types of inhibitors are called allosteric inhibitors.
![]()
Question 10.
Explain the term (i) Chemical messengers, (ii) Receptor, (iii) Antagonists, (iv) Agonists.
Answer:
(i) Chemical messengers: In the body, messages between two neurons (nerve cells) or that between neurons and muscles are communicated through a certain chemicals in substances called chemical messengers.
(ii) Receptors: Receptors are proteins that are crucial to the communication system in the body.
(iii) Antagonists: Drugs that bind to the receptor site and inhibit its natural function are called antagonists. These are useful when blocking of the message is required.
(iv) Agonists: Drugs that mimic (imitate) the natural chemical messengers by switching on the receptor are called agonists. These are useful when there is a lack of natural chemical messengers.
Question 11.
Mention the various types of chemical messengers and explain how they act?
Answer:
There are two types of chemical messengers.
(i) Hormones (ii) Neurotransmitters.
- Hormones are a group of biomolecules
which are produced in the ductless (endocrine) glands. These enter into the bloodstream and travel to different parts of the body activating all the receptors. which recognize them for message
transfer. They are not deactivated quickly. Adrenaline is an example of a hormone. - Neurotransmitters: Nerves transfer messages through neurotransmitters. These bind to the receptor (target) for a very short time to transfer the message to it and depart quickly unchanged after transferring the message. The receptor then forwards the message inside the cell. After leaving the active site, neurotransmitters undergo degradation and lose their capacity to transfer messages.
Question 12.
With reference to which classification has the statement “ranitidine, is an antacid” been given?
Answer:
This statement refers to the classification of drugs according to pharmacological effect because drugs that will be used to counteract the excess acid in the stomach will be called antacids.
Question 13.
List two major classes of antibiotics with an example of each class.
Answer:
| (a) Bactricidal | (b) Bacteriostatic |
| (i) Penicillin | (i) Erythromycin |
| (ii) Aminoglycosides | (ii) Tetracycline |
| (iii) Oxoflavin | (iii) Chloroampinicol |
![]()
Question 14.
What are tranquilizers? How do they act? Give two examples.
Answer:
Tranquilizers are neurologically active drugs. They are used in the treatment of stress anxiety, sleep disorders, and mental diseases. They act on the central nervous system by blocking the neurotransmitter dopamine in the main Dizephan, alprazolam is the examples.
Question 15.
Give two examples for each (i) Anti-inflammatory drugs (ii) Antipyretics (iii) nonsteroidal Anti-inflammatory drugs.
Answer:
(i) AntiinflammatorydrugsAcetaminophen or paracetamol, ibuprofen, aspirin.
(ii) Antipyretics: Acetylsalicylic acid (aspirin), Acetaminophen or Paracetamol.
(iii) Nonsteroidal anti-inflammatory drugs: Ibuprofen.
Question 16.
Mention an important difference between non-narcotic analgesics and narcotic analgesics.
Answer:
Non-narcotic analgesics are non-addictive drugs while narcotic analgesics are addictive drugs.
Question 17.
Give two examples of narcotic analgesics.
Answer:
Morphine and codeine are examples of narcotic analgesics.
![]()
Question 18.
Give two examples for local anesthetics.
Answer:
Procaine and Lidocaine.
Question 19.
Name the anesthetics used for major surgical procedures.
Answer:
Propofol and Isoflurane.
Question 20.
Give examples of antacids. How do antacids function in case of acidity?
Answer:
Milk of magnesia, sodium bicarbonate, aluminum hydroxide, ranitidine, cimetidine, omeprazole, rabeprazole are examples of antacid. They neutralize the acid in the stomach that causes acidity.
Question 21.
What are antihistamines? Give two examples.
Answer:
Antihistamines are drugs that provide relief to allergic effects, eg: cetirizine, levocetirizine.
Question 22.
What are antimicrobials? Give two examples.
Answer:
Drugs that are used to cure diseases caused by microbes or microorganisms such as bacteria, viruses, fungi, etc are called antimicrobials. These include antibacterials, antifungal, and viral agents.
eg: penicillin, erythromycin.
Question 123
How does (i) β lactams and (ii) macrolides function as antimicrobials?
Answer:
(i) β – lactam inhibits cell wall biosynthesis.
(ii) Macrolides target bacterial ribosomes and prevent protein production.
![]()
Question 24.
Give examples for β – lactam and macrolides antimicrobials.
Answer:
β – lactams: Penicillin, ampicillin, cephalosporins, carbapenems, and monobactams.
Macrolides: Erythromycin, azithromycin.
Question 25.
What are the uses of β-lactam and macrolide antimicrobials?
Answer:
Beta lactams: They are used to treat skin infections, dental infections ear infections, respiratory tract infections.
Macrolides: They are used to treat respiratory tract infectious, genital, gastrointestinal tract and skin infectious.
Question 26.
Give examples for ‘Fluoroquinolones’ and mention their uses.
Answer:
Clinafloxacin, ciprofloxacin, levofloxacin are examples of fluoroquinolones. They are used to treat urinary tract infections, skin infections, and respiratory infections.
Question 27.
How do tetracyclines class of antibiotics function? Mention their uses.
Answer:
Doxycycline, minocycline, oxytetracycline are examples of the tetracyclines group of antibiotics. They inhibit the bacterial protein synthesis via interaction with the 30S subunit of the bacterial ribosome.
They are used in the treatment of peptic ulcers, infection of the respiratory tract, etc.
Question 28.
What are aminoglycosides? Give examples.
Answer:
Aminoglycosides are a type of antibiotics that bind to the 30S subunit of the bacterial ribosome, thus stopping bacteria from making proteins.
eg: kanamycin, gentamycin neomycin.
![]()
Question 29.
What are food additives? Give examples.
Answer:
The substances which are not naturally a part of the food and are added to improve the quality of food are called food additives. The substances enhance the nutritive, sensory, and practical value of the food. They also increase the shelf life of food.
Important categories of food additives:
- Aroma compounds
- Food colors
- Preservatives
- Stabilizers
- Artificial Sweeteners
- Antioxidants
- Buffering substances
- Vitamins and minerals
Question 30.
What are food preservatives?
Answer:
Chemical substances which are used to protect food against bacteria, yeasts and molds are called preservatives, eg: sodium metabisulphite (sodium meta sulfite), sodium benzoate etc.
Question 31.
Name the preservative used in the preparation of pickles and vegetables.
Answer:
- Acetic acid for pickles
- Sodium meta-sulphate for fresh vegetables and fruits.
![]()
Question 32.
Name the chemicals which are used as emulsifiers.
Answer:
Sucrose esters with palmitic and steric esters are used as emulsifiers.
Question 33.
Name the physical methods used in the preservative of food.
Answer:
- Heat treatment (pasteurization and sterilizations)
- Cold treatment (chilling and freezing)
- Drying (dehydration)
- Irradiation is used to preserve food.
Question 34.
What are antioxidants? Give examples.
Answer:
Antioxidants are substances that retard the oxidative deteriorations of food. Food containing fats and oils is easily oxidized and turns rancid. To prevent the oxidation of the fats and oils, chemical BHT(butylhydroxytoluene), BHA(Butylated hydroxyanisole) are added as food additives. They are generally called antioxidants.
Question 35.
How do antioxidants prevent the oxidation of food?
Answer:
They readily undergo oxidation by reacting with free radicals generated by the oxidation of oils, thereby stop the chain reaction of oxidation of food. Sulphur dioxide and sulphites are also used as food additives. They act as anti-microbial agents, antioxidants, and enzyme inhibitors.
![]()
Question 36.
Define “Total Fatty Matter (TFM). What is its use?
Answer:
It is defined as the total amount of fatty matter that can be separated from a sample after splitting with mineral acids. The higher the TFM quantity in the soap better is its quality.
Question 37.
What is an anionic detergent?
Answer:
Anionic detergent: They are so named because a large part of their molecules are anions and it is the anionic part of the molecule is involved in the cleansing actions. These are sodium salts of sulphonated long-chain alcohols or hydrocarbon. For example, sodium lauryl sulphate, sodium dodecyl benzene sulphonate, etc. Anionic detergents are used is household work and in toothpastes.
Question 38.
What is cationic detergents?
Answer:
Cationic detergent: They are so-called because a large part of their molecule are cations and it is the cationic part of the molecule is involved in the cleansing action cationic detergents are quaternary ammonium salts of amines, with acetates, chlorides, or bromides as anions. Cetyl trimethyl ammonium bromide is a cationic detergent and is used in hair conditioners. Cationic detergents have germicidal properties and are expensive, therefore they are of limited use.
Question 39.
What is Non-ionic detergent?
Answer:
Non- ionic detergents: These detergents do not contain any ion. These are esters of high molecular weight alcohols. One such detergent is formed when stearic acid reacts with polyethylene glycol. Liquid dishwashing detergent are nonionic type.
![]()
Question 40.
Explain the terms monomer and polymer and polymerization.
Answer:
Monomer: Simple and reactive molecules from which polymers are prepared either by addition or condensation are called monomers, eg: Vinyl chloride, ethene, formaldehyde, acrylonitrile, phenol, etc.
Polymers: These are compounds of high molecular mass formed by the combination of a large number of simple molecules called monomers. The process by which monomers are converted to polymers is called polymerization.
Question 41.
What are synthetic and natural polymers? Give two examples for each type.
Answer:
Synthetic polymers are man-made polymers prepared in the laboratory, eg: polyethylene, Teflon, nylon, etc.
Natural polymers are naturally occurring polymers, mostly in plants, animals, etc. eg: Protein natural rubber, etc.
Question 42.
In which classes, the polymers are classified on the basis of molecular forces.
Answer:
On the basis of intermolecular forces of attraction operating between different polymers chains, polymers are classified as
- Elastomers
- Fibres
- Thermoplastic polymers
- Thermo setting polymers
Question 43.
Write names of monomers of the following polymers and classify them as addition or condensation polymers.
(a) Teflon, (b) Bakelite, (c) Natural rubber
Answer:
| Polymer | Type | Monomer |
| Teflon | Addition | Tetrafluoro ethene |
| Bakelite | Condensation | phenol and formaldehyde |
| Natural rubber | Addition | cis – isoprene |
![]()
Question 44.
What is the role of benzoyl peroxide in the polymerization of ethene?
Answer:
Benzoyl peroxide is an initiator. It forms a free radical.
Question 45.
What are LDPE and HDPE? How are they prepared?
Answer:
LDPE is low-density polyethylene. It is obtained by the polymerization of ethene under high pressure of 1000 – 2000 atm at 350 – 570 K in the presence of an initiator. HDPE is called high-density polyethylene. It is obtained by the polymerization of ethene in the presence of Ziegler – Natta catalyst at 333 – 343 K under 6-7 atm.
Question 46.
Write the structure of the monomers of the following polymers, (i) PVC, (ii) Polypropene, (iii) PAN, (iv) Nylon – 6.
Answer:
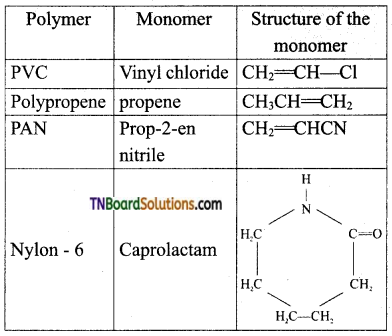
Question 47.
Give examples each of (i) addition polymers (ii) condensation polymer, (iii) copolymer.
Answer:
(i) Polythene, PVC
(ii) Buna – S, Buna – N
(iii) Nylon 6, Nylon 6, 6
![]()
Question 48.
Write the names of the structure of monomers of the following polymers, (i) Buna – S, (ii) Neoprene.
Answer:
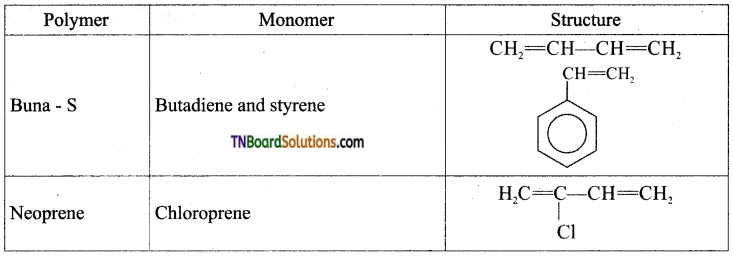
Question 49.
What is the repeating unit in the condensation polymer by combining HOOC—CH2—CFE —COOH (succinic acid) and H2NCH2CH2NH2 (ethylenediamine).
Answer:
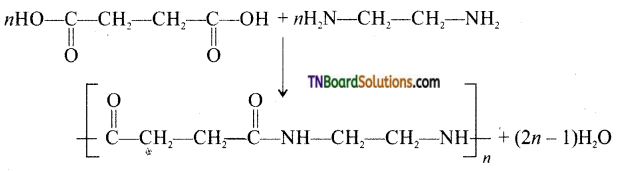
Question 50.
Differentiate between molecular structure and behavior of thermoplastic and thermosetting plastic. Give one example of each type.
Answer:
| Thermoplastic polymers | Thermosetting polymers |
| These polymers soften and melt on heating. | These polymers do not soften on heating but rather becomes hard. On prolonged heating, these start burning. |
| These polymers can be remoulded, recast and reshaped. | These polymers cannot be remoulded or reshaped |
| They are less brittle and soluble in same organic solvents. | They are move brittle and insoluble in organic solvents. |
| These polymers, usually have linear structures. | These polymers have three dimensional cross linked structures. |
| eg: Polyethylene, PVC, Teflon, Nylon etc. | eg: Bakelite, urea formaldehyde, resin, terelene etc. |
Question 51.
What are the monomeric repeating units of Nylon 6, Nylon 6, 6?
Answer:
The monomeric repeating unit of nylon 6 is  which is derived from caprolactam.
which is derived from caprolactam.
The monomer repeating unit of nylon 6, 6 is derived from two monomers hexamethylenetetramine and adipic acid.
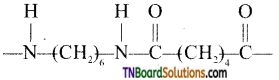
![]()
Question 52.
Name a synthetic polymer that is an amide.
Answer:
Nylon 6, 6.
Question 53.
Mention which of the following are addition polymers, (i) Terelene, (ii) Nylon 6, 6 (iii) Neoprene, (iv) Teflon.
Answer:
Neoprene and teflon are addition polymers.
Question 54.
What are biodegradable polymers?
Answer:
Polymers that disintegrate by themselves in biological systems during & certain period of time by oxidation are called biodegradable polymers, eg: PHBV. i.e., poly-β- hydroxybutyrate – co-β hydroxy valerate.
Question 55.
How are polymer classification based on forces operating between their molecule?
Answer:
Classification based as sources:
- Natural polymer: Polymers are found in nature, mostly in plants, and animals are called natural polymers, eg: proteins and natural rubber, cellulose, silk.
- Synthetic polymers: These are man-made polymers prepared in the laboratory, eg: polythene, PVC, teflon, nylon etc.
- Semi-synthetic polymers: Polymers which are obtained by making some modifications in natural polymer by artificial means, eg: Nitrocellulose, cellulose diacetate, viscose rayon etc.
![]()
Question 56.
Give the preparation of bakelite and its uses.
Answer:
Preparation of bakelite:
Monomer: Phenol and formaldehyde
Type of polymerization: Condensation polymerization.
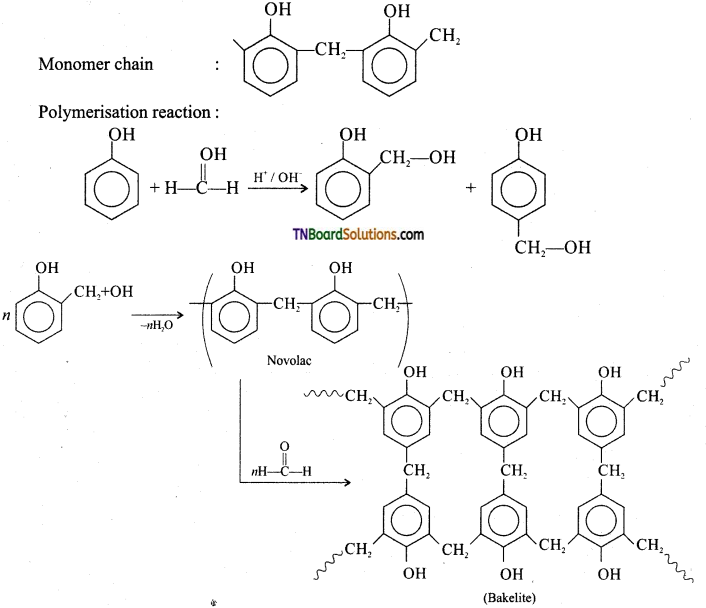
Uses: Navolac is used in paints. Soft bakelites are used for making glue for binding laminated wooden planks and in vanishes, Hard bakelites are used to prepare combs, pens etc…
Question 57.
Give the preparation and use of melamine.
Answer:
Preparation of melamine:
Monomer: Melamine and formaldehyde
Type of polymerisation: Condensation polymerisation
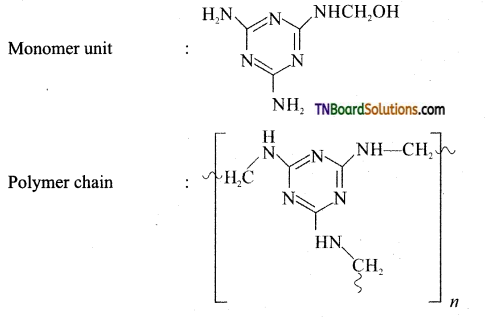
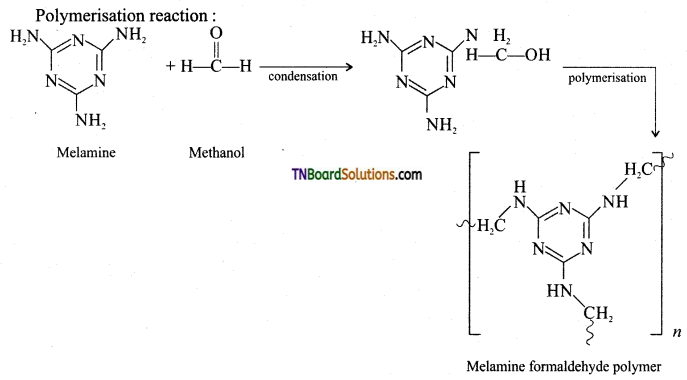
Uses: It is used for making unbreakable crockery.
![]()
Question 58.
Give examples for biodegradable polymer.
Answer:
- Polyhydroxy butyrate (PHB)
- Polyhydroxy butyrate-co-A-hydroxyl valerate (PHBV)
- Polyglycolic acid (PGA), Polylactic acid (PLA)
- Poly (∈ caprolactone) (PCL)
Question 59.
How is decron obtained from ethylene glycol and terephthalic acid?
Answer:
Decron is obtained by condensation polymerisation of ethylene glycol and terephthalic acid. The reaction is carried out at 420 – 460 K in the presence of a catalyst consisting of a mixture of zinc acetate and antimony trioxide.
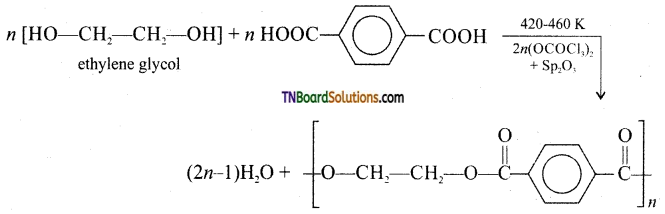
Question 60.
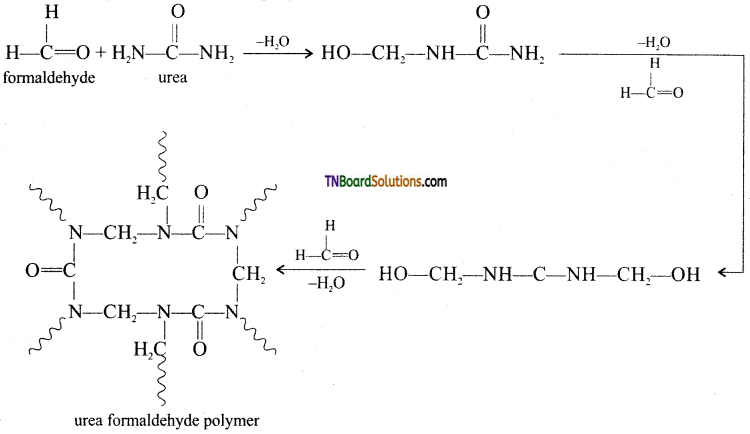
How is urea-formaldehyde prepared?
Answer:
It is formed by the condensation polymerization of the monomers urea and formaldehyde.
![]()
Question 61.
How is PHBV prepared? Give equation mention its uses.
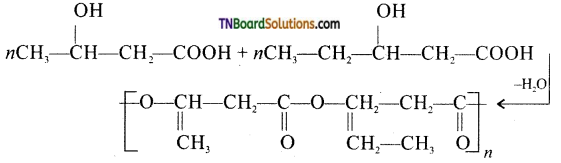
Answer:
It is the co-polymer of the monomers 3-hydroxybutyric acid and 3-hydroxypentanoic acid. In PHBV, the monomer units are joined by ester linkages.
Question 62.
Identify the type of polymer where A and B are monomers.
—A—B—B—A—A—A— B—A.
Answer:
Co-polymer.
Question 63.
Why is bakelite a thermosetting polymer?
Answer:
Due to high degree of cross linking, bakelite cannot be reshaped on heating and hence, bakelite is a thermosetting polymer.
Question 64.
Give a brief account of vulcanization of rubber.
Answer:
Vulcanization is heating natural rubber with sulphur and an appropriate additive to improve its physical properties. On vulcanization sulfur forms cross-links at the reactive sites of the double bond and thus rubber gets stiffened.
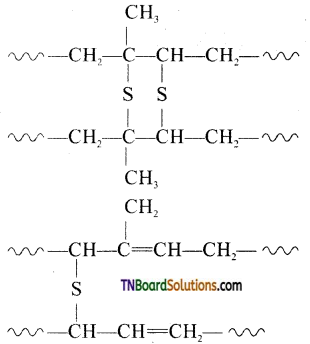
In this process, cross-linking of cis 1,4 polyisoprene chains through disulfide (—S—S) bond occurs.
![]()
Question 65.
Describe the preparation of neoprene and mention its uses.
Answer:
Neoprene is formed by the free radical polymerization of the monomer, 2-chloro buta-1,3 diene(chloroprene).
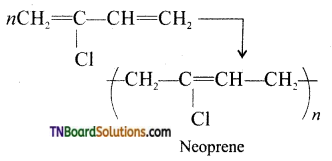
It is used is the manufacture of chemical containers and conveyor belts.
Question 66.
What is the name of the polymer formed from the monomers acrylonitrile and butal, 3-diene? How it is prepared?
Answer:
Buna – N is a copolymer formed by the polymerization of acrylonitrile and butal, 3-diene.
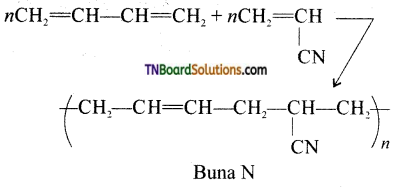
Question 67.
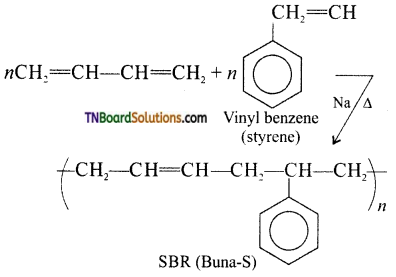
What type of polymer Buna-S is? Give its method of preparation.
Answer:
Preparation of Buna-S:
It is a copolymer. It is obtained by the polymerization of buta-1,3-diene and styrene in the ratio 3:1 in the presence of sodium.
![]()
Question 68.
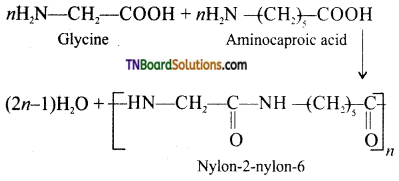
Name the polymer formed by the copolymerization of glycine and aminocaproic acid. How it is prepared?
Answer:
Nylon 2 – Nylon 6.
Preparation: By condensation polymerization of the monomers glycine and aminocaproic acid.
Choose the correct answer:
1. Which one of the following antacids is an antihistamine?
(a) Ranitidine
(b) Lansoprazole
(c) Terfen adine
(d) Luminal
Answer:
(a)
2. Which of the following is / are nurologically active drug?
(a) Aspirin
(b) Phenelzine
(c) Heroin
(d) all the above
Answer:
(d)
![]()
3. Antiseptic chloroxylenol is:
(a) 4 – chloro, 3, 5 dimethyl phenol
(b) 3 – chloro, 4, 5 dimethyl phenol
(c) 4 – chloro, 2, 5 dimethyl phenol
(d) 5 – chloro, 3, 4 dimethyl phenol
Answer:
(a)
4. Structurally a biodegradable detergent should contain a:
(a) normal alkyl chain
(b) branched alkyl chain
(c) phenyl side chain
(d) cyclohexyl side chain
Answer:
(a)
5. Which of the following statements is not correct?
(a) Some antiseptics can be added to soap.
(b) Dilute solutions of disinfectants can be used as antiseptic.
(c) Disinfectants are antimicrobial drugs.
(d) Antiseptic medicine can be infected.
Answer:
(d)
6. The most useful classification of drugs for medicinal chemists is:
(a) on the basis of chemical structure
(b) on the basis of drug action
(c) on the basis of molecular targets
(d) on the basis of active drug.
Answer:
(c)
![]()
7. A compound that causes general anti¬depressant action on the central nervous system belongs to the class of:
(a) analgesics
(b) tranquilizers
(c) narcotic analgesics
(d) antihistamines
Answer:
(b)
8. Compound which is added to soap to inpart antiseptic properties is:
(a) sodium lauryl sulphate
(b) sodium do decyl benzene sulphonate
(c) resin
(d) bithional
Answer:
(d)
9. Glycerol is added to soap. Its function is:
(a) as a filler
(b) to increase lathering
(c) to prevent rapid drying
(d) to make soap granules
Answer:
(c)
10. Polyethylene glycols are used in the preparation of which type of detergents?
(a) Cationic detergents
(b) Anionic detergents
(c) Non-ionic detergents
(d) Soaps
Answer:
(c)
![]()
11. Which of the following is employed as anti-histamine?
(a) Omeprazole
(b) Chloroampinicol
(c) Diphenylhydramine
(d) Norethindrone
Answer:
(c)
12. Tincture of iodine is:
(a) aqueous solution of I2
(b) solution of I2 in KI
(c) alcoholic solution of I2
(d) aqueous solution of KI
Answer:
(c)
13. Which among the following is not an antibiotic?
(a) Penicillin
(b) Oxytocin
(c) Erythromycin
(d) Tetracyclin
Answer:
(b)
![]()
14. Match entries in column I with appropriate entries in column II.
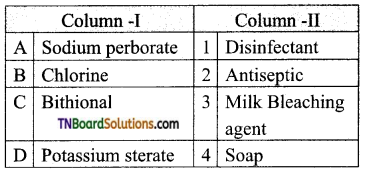
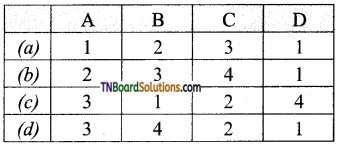
Answer:
(c)
15. Which of the following is not an antimicrobial?
(a) Salvarsan
(b) Sulphanilamide
(c) Prontsil
(d) Paracetamol
Answer:
(d)
16. Which of the following is not a semi synthetic polymer?
(a) Cis poly isoprene
(b) Cellulose nitrate
(c) Cellulose acetate
(d) Vulcanised rubber
Answer:
(a)
![]()
17. Which of the following polymers is prepared by condensation polymerisation?
(a) Styrene
(b) Nylon 6,6
(c) Teflon
(d) Rubber
Answer:
(b)
18. Which of the following is a chain growth polymer?
(a) Starch
(b) Nucleic acid
(c) Polystyrene
(d) Proteins
Answer:
(c)
19. Terelene is a condensation polymer of ethylene glycol and :
(a) benzoic acid
(b) pthalic acid
(c) salicylic acid
(d) terepthalic acid
Answer:
(d)
20. Which one of the following is a copolymer formed by condensation polymerisation?
(a) Terelene
(b) Buna – S
(c) Buna – N
(d) Neoprene
Answer:
(a)
![]()
21. Bakelite is obtained from phenol by the reaction with:
(a) HCHO
(b) (CH2OH)2
(c) CH3CHO
(d) CH3COCH3
Answer:
(a)
22. Which of the following statements is not true?
(a) B una – S is a copolymer of butadiene and styrene.
(b) Natural rubber is a 1,4-polymer of isoprene.
(c) In vulcanisation the formation of sulphur bridges between different chains makes rubber harder and stronger.
(d) Natural rubber has trans configuration at every double bond.
Answer:
(d)
23. Teflon, styron and neoprene are all:
(a) copolymers
(b) condensation polymers
(c) homo polymers
(d) monomers
Answer:
(c)
24. Which of the following sets contain only thermoplastics?
(a) Polythene, bakelite, nylon-6
(b) Glyptal, melane, PAN
(c) PVC, PMMA, Polystyrene
(d) Polypropylene urea formaldehyde, teflon
Answer:
(c)
![]()
25. Which of the following sets contain only co-polymers?
(a) SBR, Glyptal, Nylon 6,6
(b) Nylon 6, Butyl rubber, Neoprene
(c) Poly ethylene, polyester, PVC
(d) Melmac, Bakelite, Teflon.
Answer:
(a)
26. Which of the following are not thermosetting polymers?
(1) Bakelite
(2) Polystyrene
(3) PVC
(4) Melmac
(a) 1, 2
(b) 2,3
(c) 2, 4
(d) 3, 4
Answer:
(b)