Students get through the TN Board 12th Chemistry Important Questions Chapter 11 Hydroxy Compounds and Ethers which is useful for their exam preparation.
TN State Board 12th Chemistry Important Questions Chapter 11 Hydroxy Compounds and Ethers
Answer the following questions.
Question 1.
Classify the following as primary, secondary and tertiary alcohols.
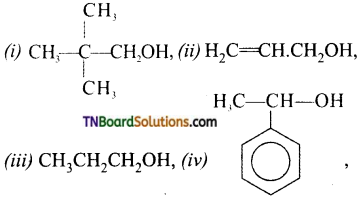
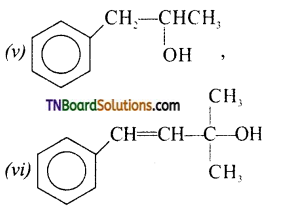
Answer:
Primary alcohols: (i), (ii), (iii)
Secondary alcohols: (iv), (v)
Tertiary alcohol: (vi)
Question 2.
Identify allyl alcohols in the above (Q.No. 1) examples.
Answer:
(ii) and (vi).
![]()
Question 3.
Name the following compounds according to IUPAC system:
Answer:
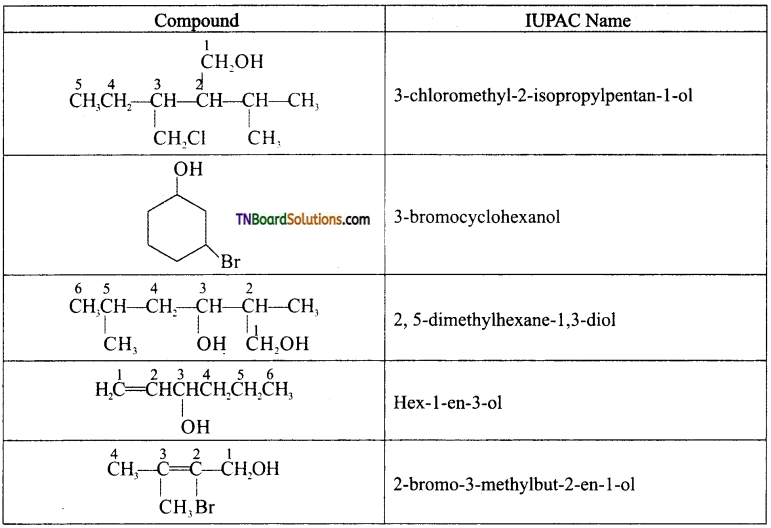
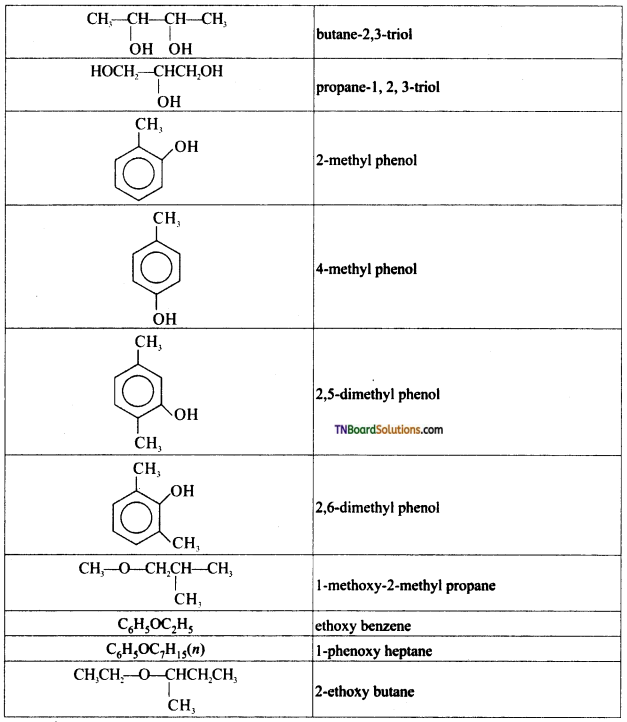
Question 4.
Write IUPAC names of the following:
Answer:
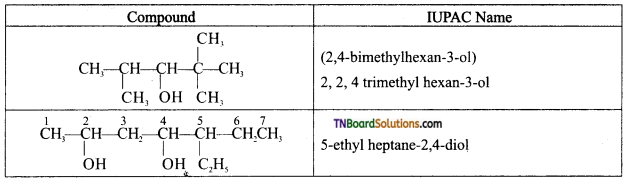
![]()
Question 5.
Write the structures of the compounds whose IUPAC names are as follows:
Answer:
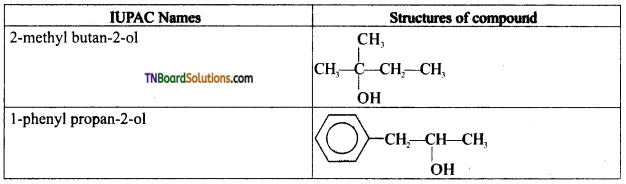
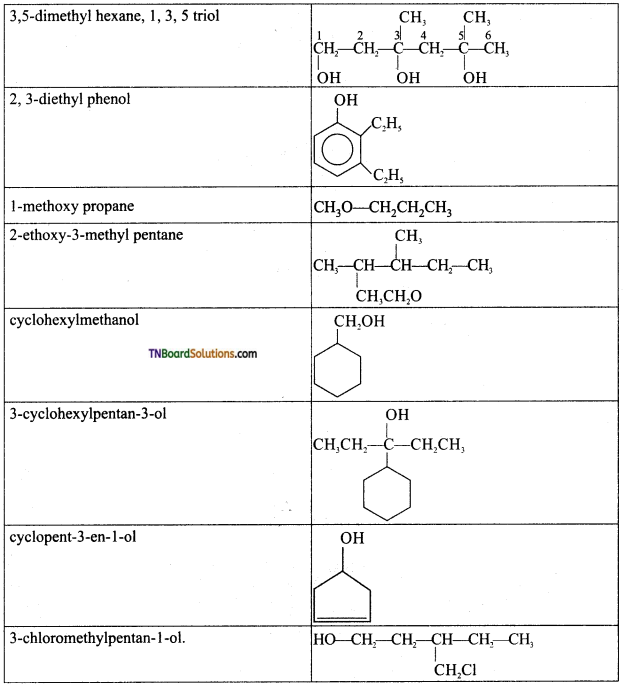
Question 6.
Complete the following reactions:
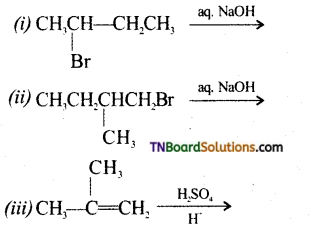
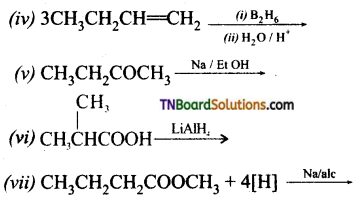
Answer:
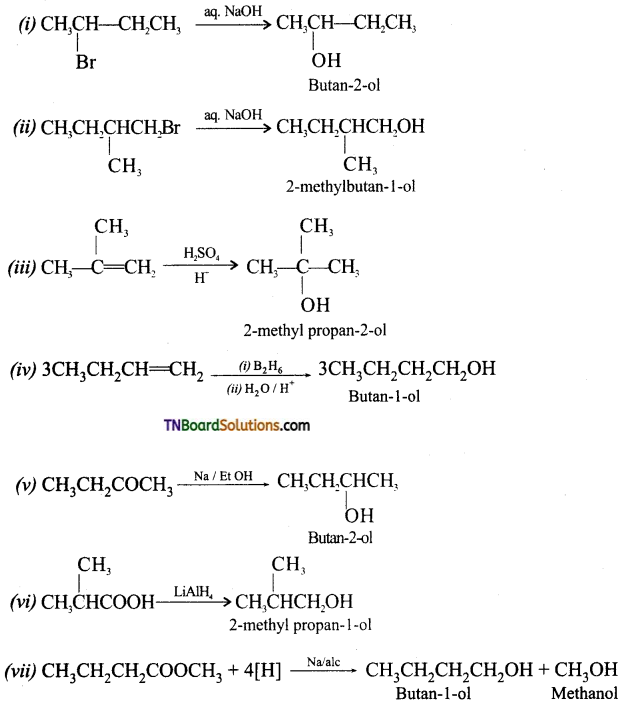
![]()
Question 7.
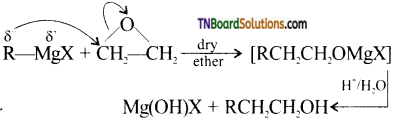
Using suitable Grignard reagent and a carbonyl compound, how will you prepare
(i) phenyl methanol,
(ii) butan-2-ol,
(iii) 2-methylhexan-2-ol,
(iv) propan-2-ol.
Answer:
(i) phenyl methanol:
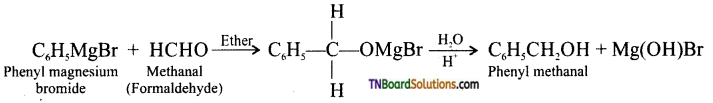
(ii) butan-2-ol:
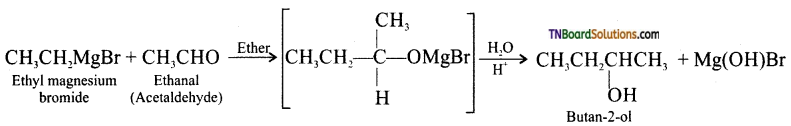
(iii) 2-methylhexan-2-ol:
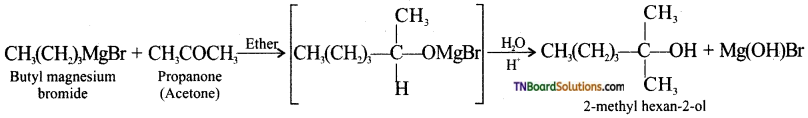
(iv) propan-2-ol:

Question 8.
Give equation for the preparation of 2-methyl-2-propanol using a suitbale Grignard reagent and a carbonyl compound.
Answer:
Tertiary containing at least two identical groups can be prepared by the addition of a Grignard reagent to an ester other the formic ester followed by acid hydrolysis.
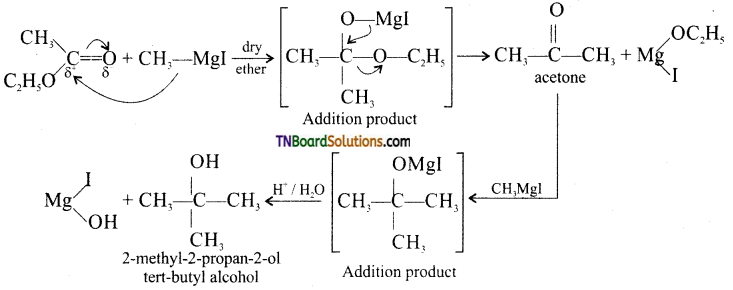
![]()
Question 9.
Name the reducing agents that will convert a carbonyl compound to an alcohol. Give an example for each.
Answer:
Raney Ni, / Sodium analgen in H2O (Na—Hg/H2O), H2 in the presence of Pt.
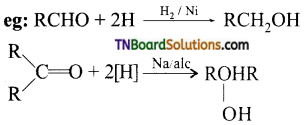
where ‘R’ is an alkyl group.
Question 10.
Why is LiAlH4 is used to reduce crotonaldehyde?
Answer:
Li crotonaldehyde is an unsaturated aldehyde (CH3CH=CHCHO). Lithium aluminium hydride selectively reduces ‘CHO’ group to ‘CH2OH’ group without affecting the C=C.
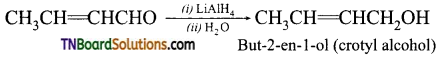
Question 11.
Name the product formed when sodium borohydride is used as a reducing agent for the reduction of a compound containing both carbonyl and carboxyl group, (or) Identify the product:
Answer:
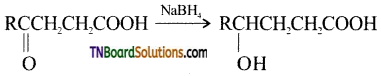
Question 12.
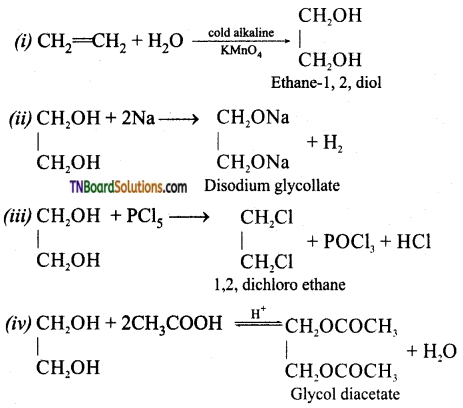
Complete the following reactions:
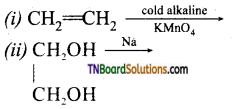
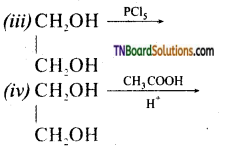
Answer:
Question 13.
How is glycerol prepared from triglycerides or fats?
Answer:
The alkaline hydrolysis of these fats gives glycerol and the reaction is known as saponification.
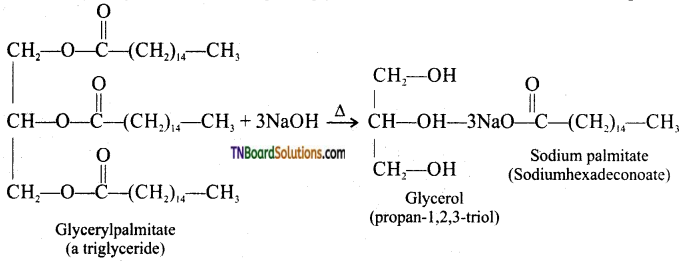
![]()
Question 14.
Complete the following equations:
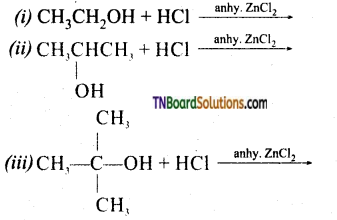
Answer:

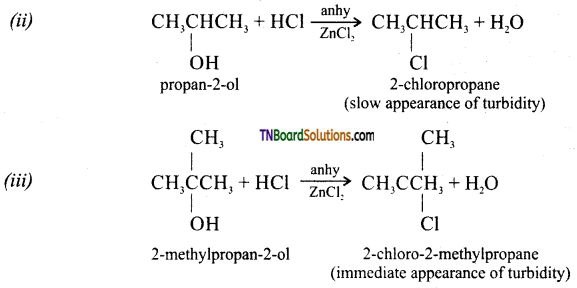
Question 15.
Give mechanism of reaction between alcohols and halogen acids.
Answer:
Primary alcohols react by SN2 mechanism while secondary and tertiary alcohol react by SN1 mechanism.
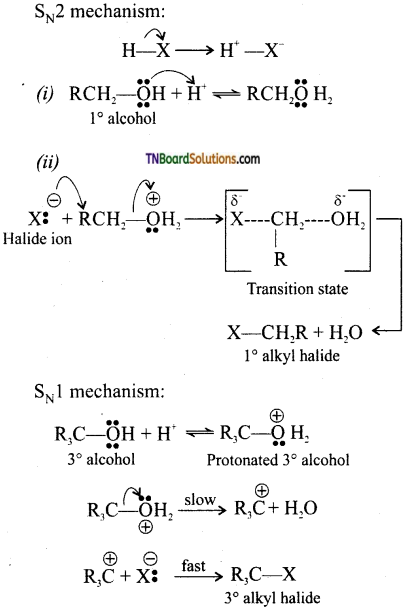
Question 16.
How will you distinguish between
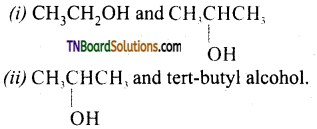
Answer:
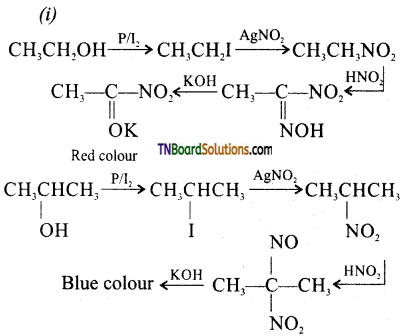
(ii) Secondary alcohol will give a blue colour as shown above whereas a tertiary alcohol will not produce any colour.
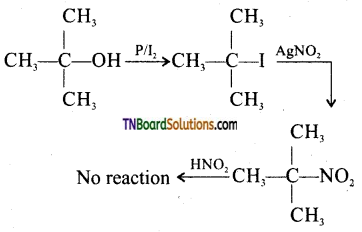
![]()
Question 17.
Give the structures of the products you would expect when each of the following alcohol reacts with (a) HCl-ZnCl2, (b) HBr, (c) SOCl2 (i) Butan-l-ol and (ii) 2-methylbutan-2-ol.
Answer:
(a) with HCl-ZnCl2 (Lucas reagent):
[2-methylbutan-2-ol being a tertiary alcohol reacts with Lucas reagent to produce turbidity immediately due to formation of insoluble tert-alkyl-halide, while butan-l-ol being a primary alcohol does not react with Lucas reagent at room temperature.]
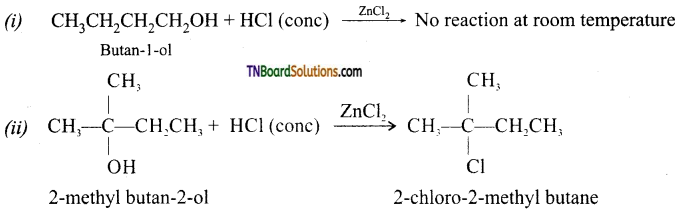
(b) with HBr: Both alcohols produce the corresponding alkyl bromides.
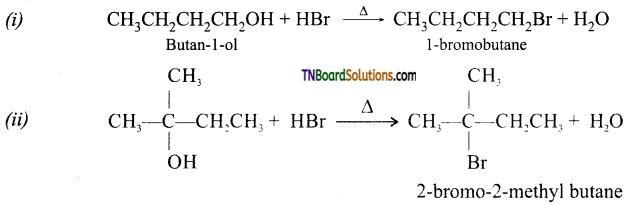
(c) with SOCl2: Both alcohols react to form their corresponding alkyl chlorides.
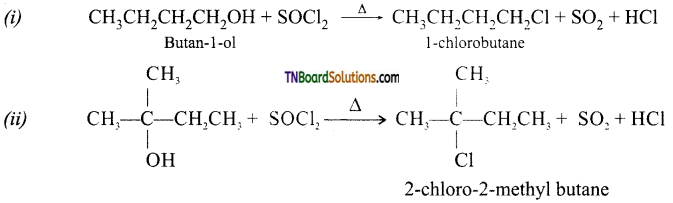
Question 18.
Arrange the following compounds in increasing order of boiling points, pentan-1 -ol, butan-1-ol, butan-2-ol, ethanol, propan-1-ol, methanol. Give reason for your answer.
Methanol < ethanol < propan-1-ol < butan-2- ol < butan-1-ol < pentan-1-ol.
Answer:
Boiling point increases regularly as the molecular mass increases due to the corresponding increase in their vander waals forces of attraction. The boiling point increases in the order.
methanol, ethanol, propan-1-ol, butan-1-ol and pentan-1-ol.
Among isomeric alcohol, 2° alcohols have lower boiling point than 1° alcohols due to corresponding decrease in the extent of hydrogen bonding because of steric hindrance. Thus the boiling point of butan-2-ol is lower than butan-1-ol.
![]()
Question 19.
Explain why propanol has higher boiling point than that of hydrocarbon butane?
Answer:
The molecuels of butane are held together by weak vander waals forces of attraction while these of propanol are held together by stronger hydrogen bonding.
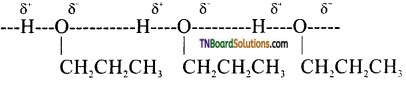
Therefore boiling point of propanol is higher than that of butane.
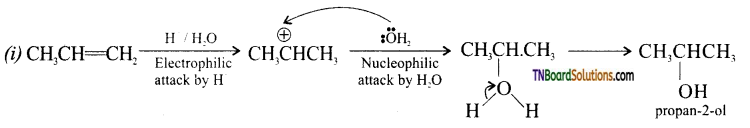
Question 20.
Write the mechanism for the reaction

Answer:
The reaction follows SN1 mechanism.
Step 1: Formation of protonated alcohol.
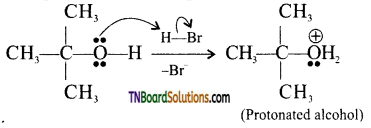
Step 2: Protonated alcohols forms carbocation.
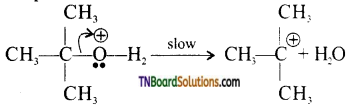
Step 3: Elimination of a proton to form alkane.
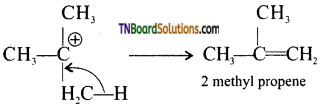
Question 21.
Give mechanism for the formation of prop-1 – ene from propan-1-ol.
Answer:
The reaction is
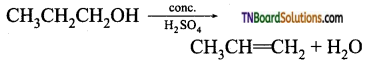
Mechanism:
Step 1: Formation of protonated alcohol.

Step 2: Formation of carbocation.

Step 3: Elimination of a proton.
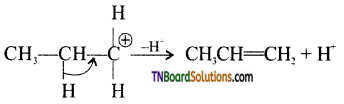
![]()
Question 22.
State Saytzeff rule.
Answer:
During intramolecular dehydration, if there is a possibility to form a carbon-carbon double bond at different locations, the preferred location is the one that gives the more (highly) substituted alkene i.e., the stable alkene.
Question 23.
Name the reagents in the following reactions:
(i) oxidation of a primary alcohol to a carboxylic acid.
(ii) oxidation of a primary alcohol to an aldehyde.
(iii) benzyl alcohol to benzoic acid.
(iv) butan-2-one to butan-2-ol.
Answer:
(i) Acidified potassium dichromate or neutral or acidic or alkaline potassium permanganate (followed by hydrolysis with dilute H2SO4).
(ii) Pyridinium chloro chromate(PCC) in CH2Cl2 or pyridinium dichromate (PDC) in CH2Cl2.
(iii) Acidified or alkaline KMnO4 (followed by hydrolysis with dil H2SO4).
(iv) Ni/H2 or NaBH4 or LiAlH4.
Question 24.
What happen when,
(i) vapours of ethanol is passed over heated copper at 573 K.
(ii) vapours of propan-2-ol is passed over heated copper at 573 K.
(iii) vapours of 2-methyl propan-2-ol is passed over heated copper at 573 K. Give equation.
Answer:
(i) Ethanal is formed.
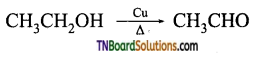
(ii) Propanone is formed.
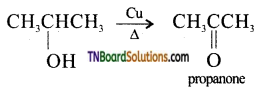
(iii) 2-methyl prop-1-ene is formed.
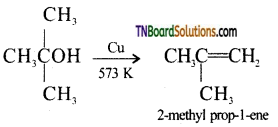
![]()
Question 25.
What is nitroglycerine? How it is formed? Give equation.
Answer:
Glycerotrinitrate (1,2,3, trinitroxy propane) is nitroglycerine. It is formed by nitration of glycerine in the presence of conc. H2SO4.
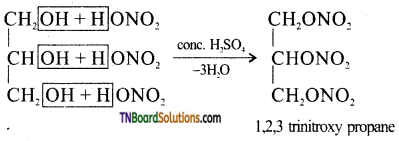
Question 26.
How is acrolein formed from glycerol?
Answer:
When glycerol is heated with dehydrating agents like conc. H2SO4, KHSO4, or P2O5 acrolein is formed.
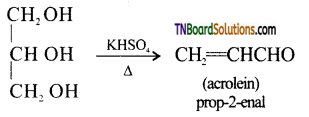
Question 27.
Mention the cause for the acidic nature of alcohols.
Answer:
The acidic character of alcohols is due to the electronegative oxygen atom which withdraws electrons of the O—H bond towards itself. As a result, the O—H bond becomes weak and hence a proton can be easily abstracted by a base, i.e., ionization of alcohol to yield alkoxide ion and H+ ions are facilitated.
ROH ⇌ RO– + H+
![]()
Question 28.
Mention the uses of (i) methanol, (ii) ethanol, (iii) ethylene glycol, (iv) glycerol.
Answer:
(i) Uses of methanol:
(a) Methanol is used as a solvent for paints, varnishes, shellac, gums, cement, etc.
(b) In the manufacture of dyes, drugs, perfumes, and formaldehyde.
(ii) Uses of ethanol:
(a) Ethanol is used as an important beverage.
(b) It is also used in the preparation of
- Paints and varnishes.
- Organic compounds like ether, chloroform, iodoform, etc.,
- Dyes, transparent soaps.
(c) As a substitute for petrol under the name power alcohol used as fuel for airplane.
(d) It is used as a preservative for biological specimens.
(iii) Uses of ethylene glycol:
(a) Ethylene glycol is used as an antifreeze in an automobile radiators.
(b) Its dinitrate is used as an explosive with DNG.
(iv) Uses of glycerol:
(a) Glycerol is used as a sweetening agent in confectionery and beverages.
(b) It is used in the manufacture of cosmetics and transparent soaps.
(c) It is used in making printing inks and stamp pad ink and lubricant for watches and clocks.
(d) It is used in the manufacture of explosives like dynamite and cordite by mixing it with china clay.
Question 29.
Alcohols are easily protonated in comparison to phenols. Explain why.
Answer:
In phenols, the lone pair of electrons on the oxygen atom is delocalized over the benzene ring due to resonance and hence, not easily available for protonation. In contrast, in alcohols, the lone pair of electrons on the oxygen atom is localized due to the absence of resonance and hence are easily available for protonation.
![]()
Question 30.
Identify the major product obtained in the following reaction:
![]()
Answer:
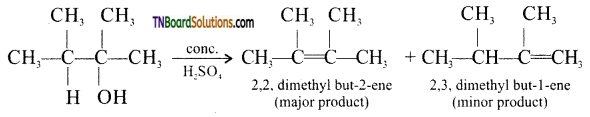
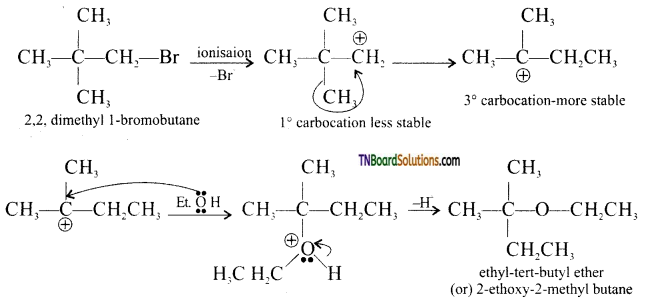
Question 31.
Identify the product of the reaction:
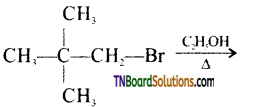
Answer:
In the presence of a weak nucleophile such as ethanol, neopentyl bromide ionizes to form first a 1° carbocation which rearranges to form a more stable 3° carbocation. This is then attacked by the weak nucleophile ethanol followed by a less proton to yield ethyl neopentyl ether.
Question 32.
Show how 2-methyl propan-1-ol is prepared by the reaction of a suitable Grignard reagent.
Answer:
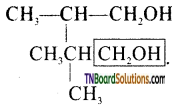
This part that has been enclosed in the box comes from methanal while the remaining part comes from the Grignard reagent. Therefore the Grignard reagent should be isopropyl magnesium bromide. Thus,
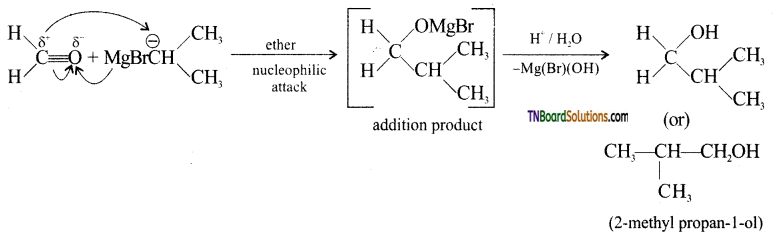
![]()
Question 33.
Write the structure of the products of the following reactions.
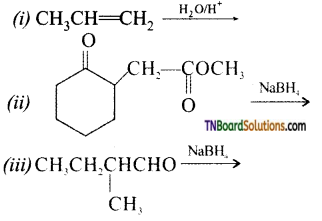
Answer:
(ii) NaBH4 (sodium bromohydride) is a reducing agent. It reduces aldehydes and ketones but not esters.
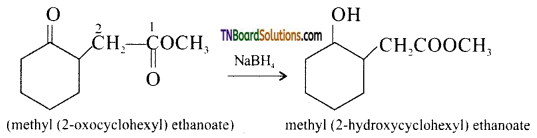
(ii) NaBH4 reduces, -CHO group to CH2OH group. Thus,
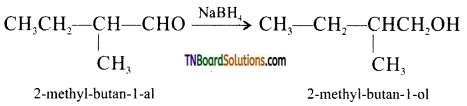
Question 34.
What is esterification? Give an example.
Answer:
Formation of an ester  from an alcohol and an organic acid in the presence of a catalyst is known as esterification. In the reaction, the ‘OH’ of the carboxylic acid and ‘H’ of the alcoholic group are removed as water.
from an alcohol and an organic acid in the presence of a catalyst is known as esterification. In the reaction, the ‘OH’ of the carboxylic acid and ‘H’ of the alcoholic group are removed as water.
Eg:
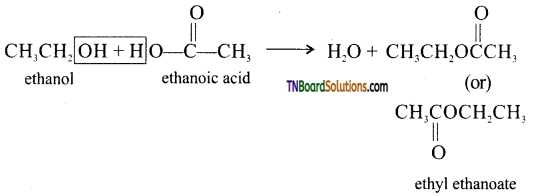
![]()
Question 35.
Why are alcohols are weaker acids than water?
Answer:
In water, ‘O’ atom is attached to two ‘H’ atom, but in alcohols, ‘O’ atom is attached to one ‘H’ atom and one ‘R’ group (alkyl group). Since ‘R’ group has an electron-donating inductive effect (+I), it increases the electron density in the O—H bond compared to that O—H bond in water. In other words, —O—H bond in alcohols is stronger than the —O—H bond in water. As a result, proton removal from alcohol is more difficult than in water. Hence, alcohols are weaker acids than water.
Question 36.
The acidic nature of alcohols is in the order 1° alcohol > 2° alcohol > 3° alcohol. Explain.
Answer:
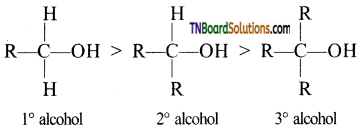
Alkyl (R) groups are electron releasing (i.e., +I effect) the electron density in the O—H bond in tertiary alcohols is maximum followed by secondary alcohol, while in primary alcohols, it is minimum. This means that O—H bond in tertiary alcohol is the strongest and hence most difficult to break followed by O—H bond in the secondary alcohols, while the O—H bond in primary alcohols is the weakest and hence most easy to break. Thus the acidic strength is in the order.
Primary > Secondary > Tertiary
Question 37.
Arrange the following compounds in increasing order against each.
(i) CH3CH2OH, CF3CH2OH. CCl3CH2OH (acid strength)
(ii) 2-methyl-2-propanol, 1-butanol, 2-butanol (reactivity towards sodium)
Answer:
(i) Due to -I effect of halogens, the electron density in the O—H bond decreases. As a result, the O—H bond strength becomes weak and proton removal is easier compared to CH3CH2OH. Further, since fluorine has a stronger -I effect, than chlorine, CF3CH2COOH is a stronger acid than CCl3CH2COOH. Hence, CF3CH2OH is the strongest acid while CH2CH2OH is the weakest acid. Hence increasing order of acid strength is
CH3CH2OH < CCl3CH2OH < CF3CH2OH.
(ii) It is an acid-base reaction since alcohols are acidic and sodium is a strong base. As such, the reactivity of these alcohols towards sodium increases as the acidic character of alcohols increases. Since the acidic character increases in the order 3° < 2° < 1°. Therefore the reactivity towards alcohol increases in the same order.
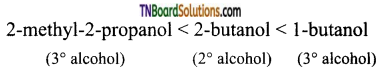
![]()
Question 38.
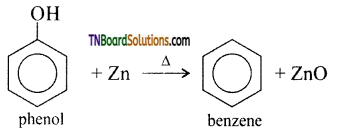
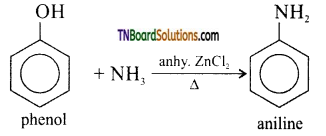
How will you convert phenol to (i) benzene, (ii) aniline, (iii) phenylacetate, (iv) phenyl benzoate, (v) anisole?
Answer:
(i) Benzene: Phenol is converted to benzene on heating with zinc dust. In this reaction, the hydroxyl group which is attached to the aromatic ring is eliminated.
(ii) Aniline: Phenol on heating with ammonia in presence of anhydrous ZnCl2 gives aniline.
(iii) Phenyl acetate:
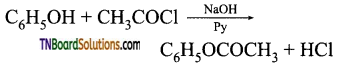
(iv) Phenyl benzoate:
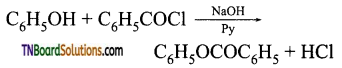
(v) Anisole:
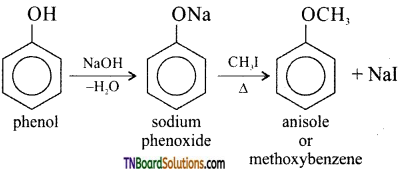
Question 39.
Identify A and B in the following:
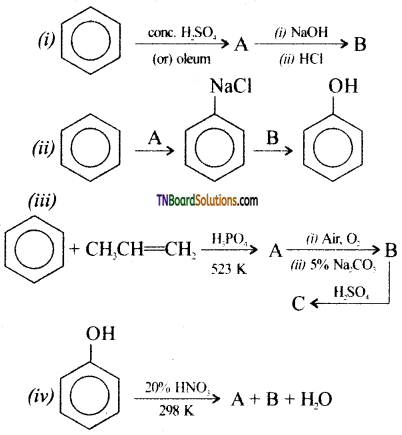
Answer:
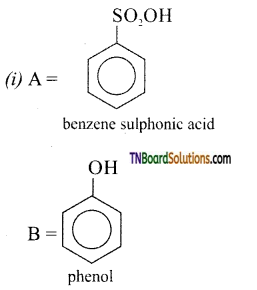
(ii) A= HNO2 (273 -278) K
B = H2O
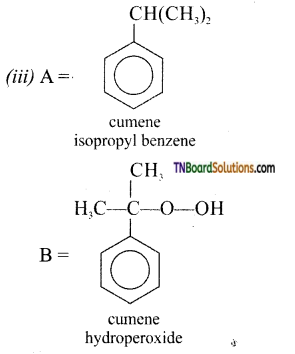
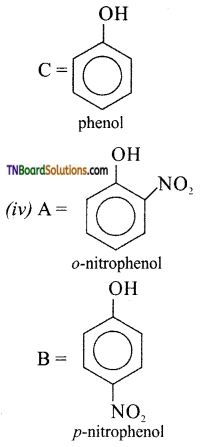
![]()
Question 40.
What is picric acid? How it is prepared? 2,4,6 trinitrophenol is called picric acid.
Answer:
Nitration with cone. HNO3 and conc.H2SO4 gives picric acid.
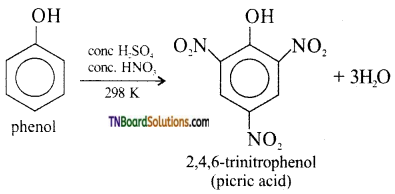
Question 41.
Give equation for the following:
(i) nitration of phenol,
(ii) sulphonation of phenol,
(iii) nitrosation of phenol.
Answer:
(i) Nitration of phenol: Phenol can be nitrated using 20% nitric acid even at room temperature, a mixture of ortho and para nitro phenols are formed.
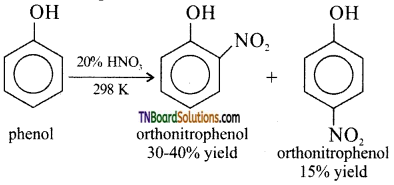
(ii) Sulphonation of phenol: Phenol reacts with cone. H2SO4 at 280 K to form o-phenol sulphonic acid as the major product. When the reaction is carried out at 373 K the major product is p-phenol sulphonic acid.
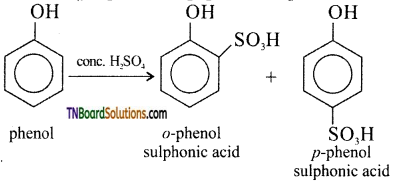
(iii) Nitrosation of phenol: phenol can be readily nitrosated at low temperature with HNO2.
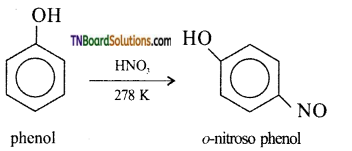
![]()
Question 42.
What happens when phenol is treated with
(i) acidified potassium dichromate,
(ii) hydrogen in the presence of nickel as a catalyst,
(iii) bromine water,
(iv) bromine in the presence of CCl4.
Answer:
Phenol treated with
(i) acidified potassium dichromate (oxidation):
Phenol undergoes oxidation with air or acidified K2CrO7 with conc. H2SO4 to form 1, 4-benzoquinone.
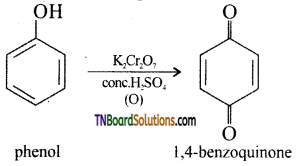
(ii) hydrogen in the presence of nickel as catalyst (reduction): Phenol on catalytic hydrogenation gives cyclohexanol.
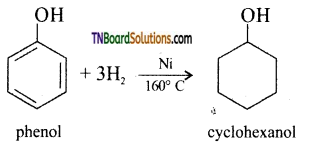
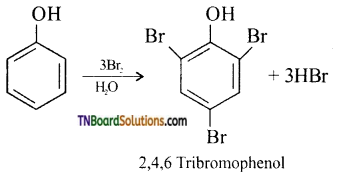
(iii) bromine water (halogenation): phenol reacts with bromine water to give a white precipitate of 2,4,6-tribromo phenol.
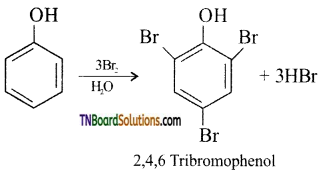
(iv) bromine in the presence of CCl4: If the reaction is carried out in CS2 or CCl4 at 278 K, a mixture of ortho and para bromo phenols are formed.
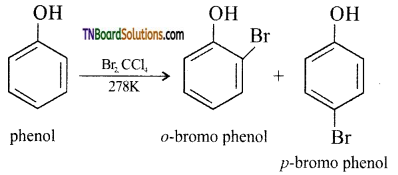
Question 43.
Explain why the phenolic group is ortho para directing.
Answer:
The lone pair of electrons on the oxygen atom of the phenolic group enter into conjugation with the benzene ring due +M effect of the phenolic group. As a result, the ortho and para position become electron-rich compounds to the meta position. Hence the electrophilic attack the ortho and para position.
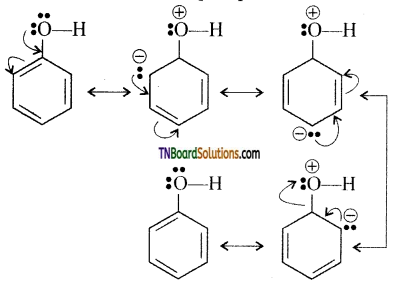
![]()
Question 44.
Write a short note on Reimer-Tiemann reaction.
Answer:
On treating phenol with CHCl3 / NaOH, a -CHO group is introduced at the ortho position.
This reaction proceeds through the formation of substituted benzal chloride intermediate.
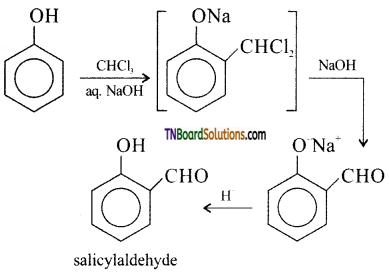
Question 45.
How will you distinguish between alcohol and phenol?
Answer:
- Phenol reacts with benzene diazonium chloride to form a red-orange dye, but ethanol has no reaction with it.
- Phenol gives purple coloration with neutral ferric chloride solution, alcohols do not give such coloration with FeCl3.
- Phenol reacts with NaOH to give sodium phenoxide. Ethyl alcohol does not react with NaOH.
Question 46.
Mention the uses of phenol.
Answer:
- About half of the world production of phenol is used for making phenol-formaldehyde resin. (Bakelite).
- Phenol is a starting material for the preparation of
(a) drugs such as phenacetin, salol, aspirin, etc.
(b) phenolphthalein indicator.
(c) explosive like picric acid. - It is used as an antiseptic-carbolic lotion and carbolic soaps.
![]()
Question 47.
Give the IUPAC names of the following ethers.
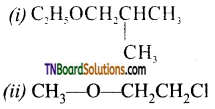
Answer:
(i) 1-ethoxy-2-methyl propane
(ii) 2-chloro-l-methoxy ethane
(iii) 4-nitroanisole
(iv) 1-methoxy propane
(v) 4-ethoxy-1, 1, dimethyl cyclohexane
(vi) ethoxy benzene
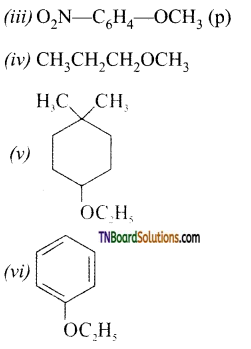
Question 48.
Ethers are soluble in water. Give reason.
Answer:
The oxygen of ether can also form hydrogen bonds with water and hence they are miscible with water. Ethers dissolve a wide range of polar and non-polar substances.
Question 49.
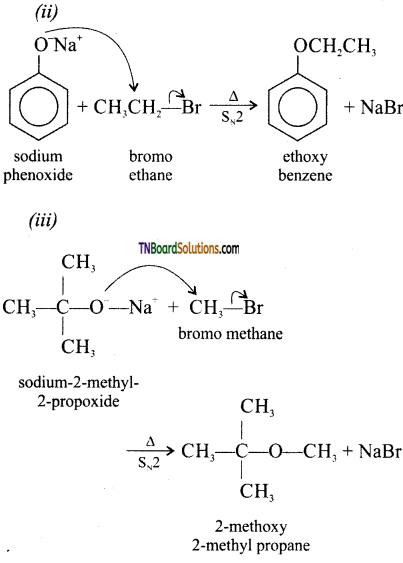
Write the names of reagents and equations for the preparation of the following ethers by Williamson’s synthesis.
(i) 1 -propoxypropane
(ii) Ethoxy benzene
(iii) 2-methyl-2-methoxy propane
(iv) 1-methoxyethane
Answer:
(i)
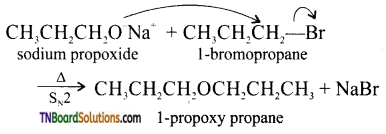
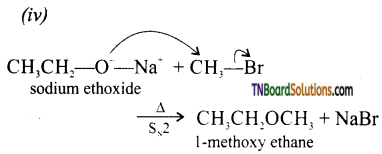
![]()
Question 50.
Give the mechanism for the conversion of (i) ethanol to ethoxyethane, (ii) dehydration of 1-propanol to 1-proproxy propane.
Answer:
(i)
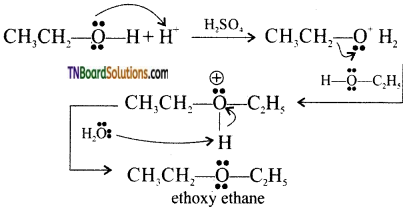
[Acid catalyzed 1° alcohol to ethers occurs via SN2 reaction, involving a nucleophilic attack by the alcohol molecule on the protonated alcohol molecule.]
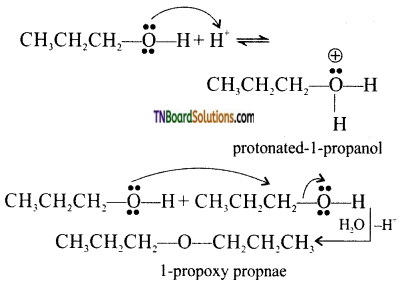
Question 51.
Explain why the boiling point of ethers is slightly higher than that of alkanes and lower than that of alcohols of comparable molecular mass.
Answer:
Due to weak dipole-dipole interactions, the boiling point of ethers is only slightly higher than those of u-alkanes having comparable molecular masses.
The boiling points of ethers are less than that of alcohols is that ethers do not form hydrogen bonds.
Question 52.
Mention the uses of (i) Diethyl ether and (ii) Anisole.
Answer:
Uses of Diethyl ether:
- Diethyl ether is used as a surgical anesthetic agent in surgery.
- It is a good solvent for organic reactions and extraction.
- It is used as a volatile starting fluid for diesel and gasoline engines.
- It is used as a refrigerant.
Uses of anisole:
- Anisole is a precursor to the synthesis of perfumes and insecticide pheromones
- It is used as a pharmaceutical agent.
![]()
Question 53.
Discuss the mechanism of the reaction
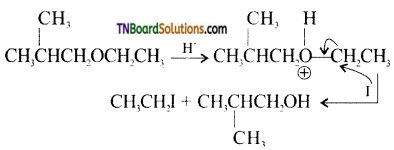
CH3OCH2CH3 + HI → CH3I + CH3CH2OH.
Answer:
This reaction is an SN2 reaction. The cleavage of ethers by halogen acids occurs by the following mechanism.
Step 1: Ethers being Lewis acids, undergo protonation to form oxonium ions.
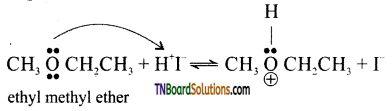
This reaction takes place with HBr or HI because they are sufficiently acidic.
Step 2: Iodide ion is a good nucleophile. Due to steric hindrance, it attacks the smaller alkyl group of oxonium ion formed in step 1 and displaces the alcohol molecule by SN2 mechanism as shown below:
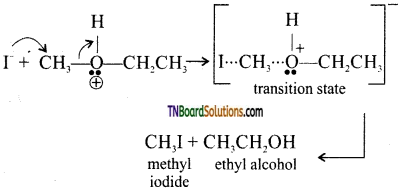
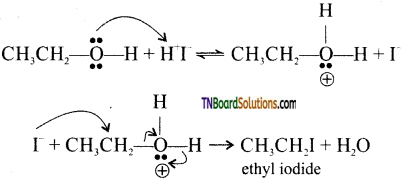
Step 3: when excess HI is used, ethanol formed reacts with another molecule of HI to form ethyl iodide.
Question 54.
Explain why ‘OCH3’ in the anisole group is ortho-para directing.
Answer:
The lone pair of electrons on the oxygen atom of the alkoxy group enters into the conjugation with the benzene ring due to its +M effect.
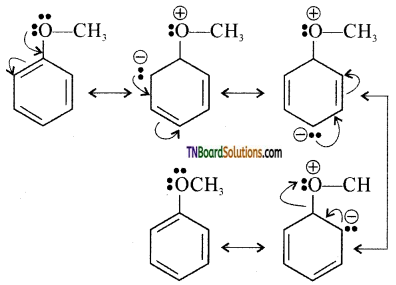
As a result, the ortho and para position become rich in electron density compared to the meta position. Hence, the electrophile attacks ortho and para position.
![]()
Question 55.
Briefly account of dipolar nature of C—O bonds in ethers.
Answer:
Because of the greater electronegativity of oxygen and carbon, the C—O bond is slightly polar thus have a dipole moment. Since the two C—O bonds in ethers are inclined to each other at an angle of 110°, the two dipoles do not cancel each other. As a result, ethers have a dipole moment of 1.15 to 1.3D

Question 56.
Give the equation for the reaction between diethyl ether with an excess of oxygen.
Answer:

Question 57.
Give examples for (i) phthalein fusion and (ii) coupling reaction.
Answer:
(i) Phthalein fusion: On heating phenol with phthalic anhydride in presence of conc. H2SO4, phenolphthalein is obtained.
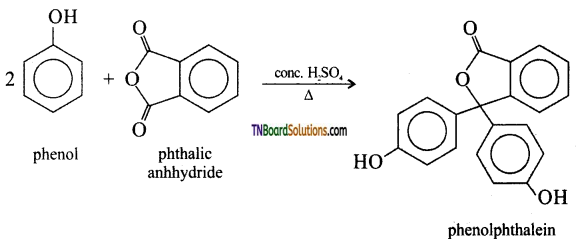
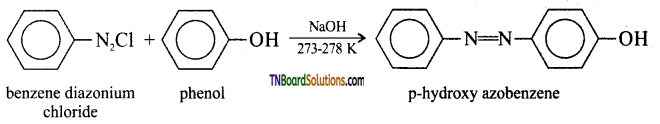
(ii) Coupling reaction: Phenol couples with benzene diazonium chloride in an alkaline solution to form p-hydroxy azobenzene (a red-orange dye).
Choose the correct answer:
1. The IUPAC name of the compound is:

(a) 2-chloro-5- hydroxyhexane
(b) 2-hydroxy-5-chlorohexane
(c) 5-chlorohexan-2-ol
(d) 2-chlorohexan-5-ol
Answer:
(c)
![]()
2. The IUPAC name of the compound is:

(a) 1-methoxy-1-methyl ethane
(b) 2-methoxy-2-methyl ethane
(c) 2-methoxypropane
(d) isopropyl-methyl ether
Answer:
(c)
3. Arrange the following compounds in increasing order of boiling point:
I – propan-1-ol
II – butan-1-ol
III – butan-2-ol
IV – pentan-1-ol
(a) I, III, II, IV
(b) I, II, III, IV
(c) IV, III, II, I
(d) IV, II, III, I
Answer:
(a)
Hint: The boiling point increases as the molecular mass increases. Further among isomeric alcohols 1° alcohols have higher boiling points than 2° alcohols.
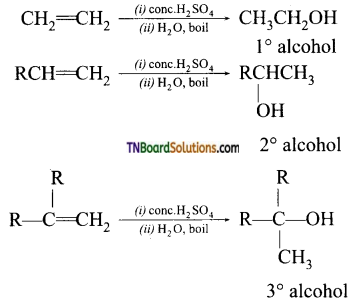
4. Acid catalysed hydration of alkenes except ethene, leads to the formation of:
(a) primary alcohol.
(b) secondary or tertiary alcohol.
(c) a mixture of primary and secondary alcohol.
(d) a mixture of secondary and tertiary alcohol.
Answer:
(b)
Hint: Hydration of ethene gives a 1° alcohol while all others give a either a 2° alcohol or 3° alcohol.
![]()
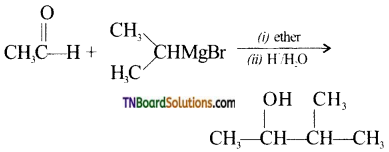
5. Which of the following Grignard reagent is suitable for the preparation of 3-methyl-2- butanol?
(a) 2-butanone + methyl magnesium bromide
(b) Acetone + ethyl magnesium bromide
(c) Acetaldehyde + isopropyl magnesium bromide
(d) Ethyl propionate + methyl magnesium bromide
Answer:
(c)
Hint:
6. Which of the following esters shown, after reduction with LiAlH4 and aqueous workup, will yield two molecules of only a single alcohol?
(a) C6H5COOC6H5
(b) CH3CH2COOCH2CH3
(c) CH3COOCH3
(d) C6H5COOCH2C6H5
Answer:
(d)
Hint: Acyl and arylalkyl groups contain the same number of carbon atoms.
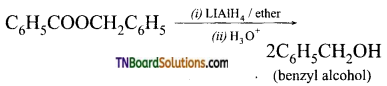
7. Which of the following will exhibit highest boiling point?
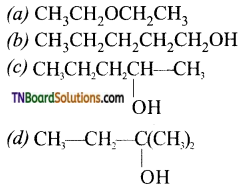
Answer:
(b)
Hint: Among isomeric alcohols, the alcohol with no branching has the highest boiling point.
![]()
8. Consider the reactions:
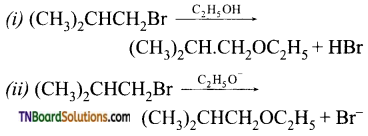
The mechanisms of reactions (i) and (ii) are respectively:
(a) SN1 and SN2
(b) SN1 and SN1
(c) SN2 and SN2
(d) SN2 and SN1
Answer:
(c)
9.

which of the following compounds will be formed?
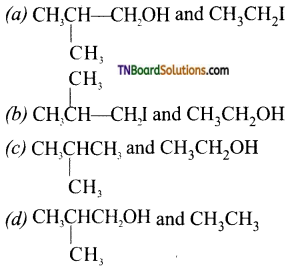
Answer:
(a)
Hint: Nucleophilic attack occurs on the smaller alkyl group.
![]()
10. The reaction of  with RMgX leads to the formation of:
with RMgX leads to the formation of:

Answer:
(d)
Hint:
11.
![]()
X is:
(a) bromine in benzene
(b) bromine in water
(c) potassium bromide solution
(d) bromine in CCl4 at 0° C
Answer:
(b)
Hint:
12. Phenol can be distinguished from ethanol by the following reagent except.
(a) sodium
(b) I2 / NaOH
(c) neutral FeCl3
(d) Br2 / H2O
Answer:
(a)
Hint: Na reacts with both ethapol and phenol.
![]()
13. An ether is more volatile than an alcohol having the same molecular formula. This is due to:
(a) intermolecular hydrogen bonding in alcohols
(b) dipolar character of ethers
(c) alcohols having resonance structures
(d) intermolecular hydrogen bonding in ethers
Answer:
(a)
14. Mark the correct increasing order of reactivity of the following compounds with HBr / HI.
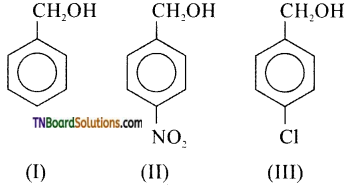
(a) I < II < III
(b) II < I < III
(c) II < III < I
(d) III < II < I
Answer:
(c)
Hint: All the three benzyl alcohols react with HBr or HI through the formation of intermediate carbocation. Obviously more stable the carbocation, more reactive is the alcohol. Now, electron withdrawing group i.e, NO2, Cl, etc., decrease the stability of carbocations. Since, NO2 is a stronger, electron withdrawing group, then Cl, stability of carbocation increases in the order.
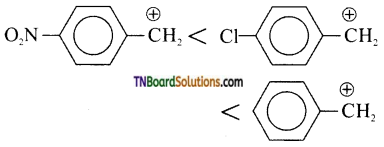
Therefore, the reactivity of the benzyl alcohols increases in the same orders, i.e., II < III < I.
![]()
15. Which of the following reagents can be used to oxidize primary alcohols to aldehydes?
1. CrO3 in acidic medium,
2. KMnO4 in acidic medium,
3. Pyridinium chlorochromate,
4. Heat in the presence of Cu at 573 K.
(a) 1, 3, 4
(b) 1, 3
(c) 1, 4
(d) none of the above
Answer:
(a)
Hint: KMnO4 will oxidise the aldehyde formed to carboxylic acid.
16. Assertion: Bond angles in ethers is slightly more than the tetrahedral angle.
Reason: There are repulsions between two bulky ‘R’ groups.
(a) Assertion and reason both are correct, and reason is the correct explanation of assertion.
(b) Assertion and reason both are correct, but reason is not the correct explanation of assertion.
(c) Assertion is wrong, but reason is correct.
(d) Both assertion and reason are wrong.
Answer:
(c)
Hint: Correct assertion: Bond angles in ethers are slightly more than the tetrahedral bond angle.
17. Assertion: Bromination of benzene does not require Lewis acid is catalyst.
Reason: Lewis acid polarises the bromine molecule in Friedel craft’s reaction.
(a) Assertion and reason both are correct, and reason is the correct explanation of assertion.
(b) Assertion and reason both are correct, but reason is not the correct explanation of assertion.
(c) Assertion is true, but reason is wrong.
(d) Both assertion and reason are wrong.
Answer:
(b)
![]()
18. Match the entries in column I with appropriate entries in column II and choose the correct option using the codes given:
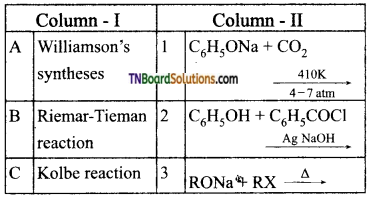
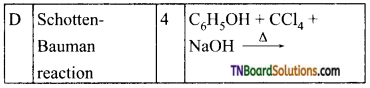
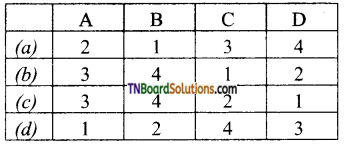
Answer:
(b)
19. Match the entries in column I with appropriate entries in column II and choose the correct option using the codes given:
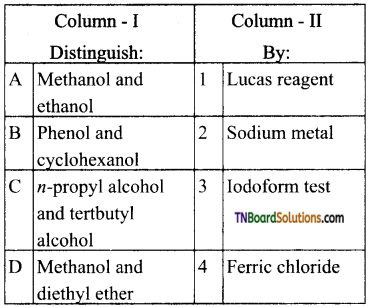
Answer:
(d)
![]()
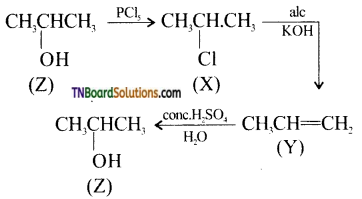
20. In the following sequence of reactions,

(a) CH3CH2CH2OH
(b) CH3CH(OH)CH3
(c) CH3CH2CH2CH2OH
(d) (CH3)3CCH2OH
Answer:
(b)
Hint: Since alkenes give Markovnikoff addition products, therefore 1° alcohols (a), (c) and (d) cannot be obtained by hydration. Thus, ‘Z’ is 2-propanol.
i.e.
21. Consider the following reaction:
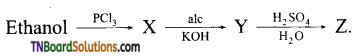
The compound ‘Z’ is:
(a) CH3CH2OCH2CH3
(b) CH3CH2OSO3H
(c) CH3CH2OH
(d) CH2=CH2
Answer:
(c)
Hint:
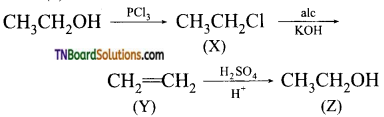
22. Formation of tert-butyl ether by reaction of sodium tert-butoxide and methyl bromide involves:
(a) elimination reaction
(b) electrophilic addition reaction
(c) nucleophilic addition reaction
(d) nucleophilic substitution reaction
Answer:
(d)
![]()
23.
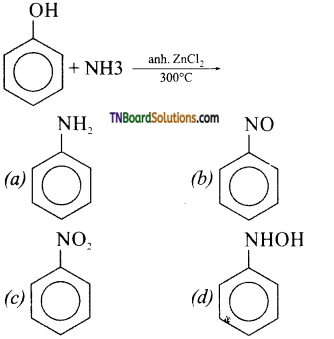
Answer:
(a)
24. The red coloured compound formed during Victor-Meyer’s test is:
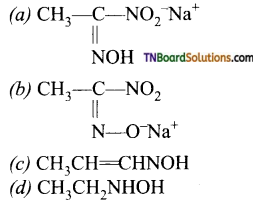
Answer:
(b)
Hint:
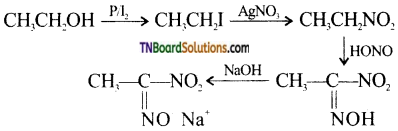
![]()
25. Following compounds are given:
(i) CH3CH2OH
(ii) CH3COCH3
(iii) CH3CH(OH)CH3
(iv) CH3OH
Which of the above compounds on being warmed with iodine solution andNaOH, will give iodoform?
(a) (i), (iii) and (iv)
(b) only (i)
(c) (i), (ii) and (iii)
(d) (i) and (ii)
Answer:
(c)
Hint: Compounds containing CH3CO group or CH3CH(OH) group on warming with I2 and NaOH will form iodoform. Hence, (i), (ii) and (iii) will give iodoform.