Students get through the TN Board 12th Chemistry Important Questions Chapter 13 Organic Nitrogen Compounds which is useful for their exam preparation.
TN State Board 12th Chemistry Important Questions Chapter 13 Organic Nitrogen Compounds
Answer the following questions.
Question 1.
Classify the following aliphatic or aromatic nitro compounds.
(i) 1, 2, dimethyl-1-nitropropane
(ii) 2-nitro-1 -methyl benzene
(iii) 1, 3, 5 trinitro benzene
(iv) 2-phenyl-1-nitro ethane
(v) 2-methyl-2-nitro propane
Answer:
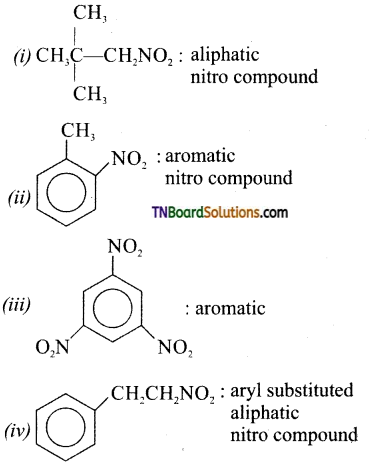

![]()
Question 2.
What are nitro compounds? How are they classified? Give one example for each type.
Answer:
(i) Nitro compounds are considered as the derivaties of hydrocarbons. If one of the hydrogen atom of hydrocarbon is replaced by the —NO2 group, the resultant organic compound is called a nitrocompound.
(ii) Examples for primary nitro compound.
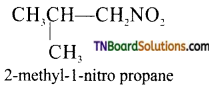
(iii) Example for secondary nitro compound.
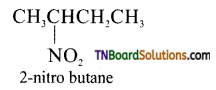
(iv) Example for a tertiary nitro compound.
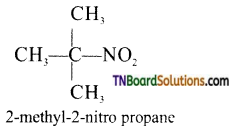
Question 3.
Write the structural formula of the isomers of the following compound and indicate the type of isomerism involved, (i) 1-nitrobutane,
(ii) nitroethane.
Answer:
(i) (a) 1-nitrobutane and 2-methyl-1-nitropropane are- chain isomers. These two isomers differ in the length of carbon chain and hence exhibit chain isomerism.

(b) 1-nitrobutane, 2-nitrobutane and 2-methyl 2-nitropropane are position isomers. They differ in the position of the functional group and hence exhibit positfon isomerism.
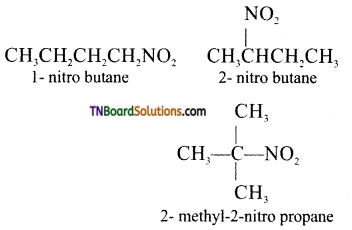
(c) 1-nitrobutane and butyl nitrite are functional isomers. They differ in the nature of the functional group and hence functional isomerism.

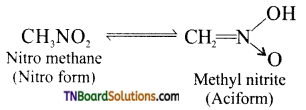
(ii) Nitromethane and methyl nitrite exhibit tautomerism.
![]()
Question 4.
Methyl nitrite and nitro methane exhibits tautomerism. How will you distinguish between these form?
Answer:
| Nitro form | Aci-form |
| Less acidic | More acidic and also called pseudoacids (or) nitronic acids. |
| Dissolves in NaOH slowly. | Dissolves in NaOH instantly. |
| Decolourises FeCl3 solution. | With FeCl3 gives reddish-brown colour |
| Electrical conductivity is low. | Electrical conductivity is high. |
Question 5.
Between 2-nitrobenzene, and 2-methyl – 2- nitro propane which does not exhibit tautomerism? Why?
Answer:
Tautomerism is exhibited by nitro alkanes which contain one or more ‘α’ hydrogen atoms. Then 1° and 2° nitro alkanes exhibit tautomerism while 3° nitro alkanes which do not have a ‘α’ hydrogen atom does not exhibit tautomerism.
2-nitrobutane has a hydrogen atom and hence exhibit tautomerism. While 2-methyl -2- nitropropane does not contain an a hydrogen atom and hence does not exhibit tautomerism.
Question 6.
Explain the acidic nature of nitroalkanes.
Answer:
Primary and secondary nitro alkanes show acidic nature because of the presence of a hydrogen atom.
Because of the presence of electron withdrawing nitro group, the ‘α’ hydrogen atom is abstracted by a base.
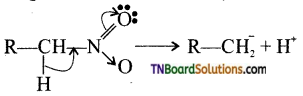
Hence, they are acidic. When the number of alkyl groups attached to α- carbon acidity decreases because of +1 effect of alkyl groups.

Question 7.
How will you prepare nitro benzene from (i) ethyl bromide (ii) methane, (iii) α – chloroacetic acid (iv) tert-butyl amine, (v) acetaldoxine.
Answer:
(i) Alkyl bromides (or) iodides on heating with ethanolic solution of potassium nitrite gives nitroethane.
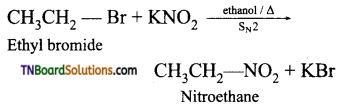
(ii) Gaseous mixture of methane and nitric acid passed through a red hot metal tube to give nitromethane.
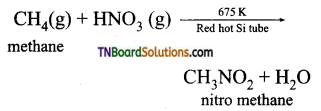
(iii) α – chloroacetic acid when boiled with aqueous solution of sodium nitrite gives nitromethane.
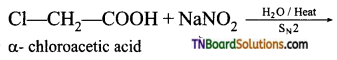
![]()
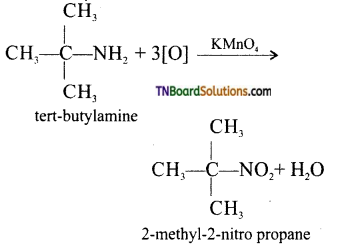
(iv) tert-butyl amine is oxidised with aqueous KMnO4 to give tert – nitro alkanes.
(v) Oxidation of acetaldoxime and acetoneoxime with trifluoroperoxy acetic acid gives nitroethane (1°) and 2- nitropropane (2°) respectively.
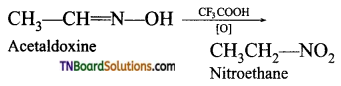
![]()
Question 8.
Give the equation for the reduction of nitromethane in (a) acid medium and (b) neutral medium.
Answer:
(a) Nitromethane on reduction in acid medium gives methylamine.

(b) Reduction under neutral condition using Zn / NH4OH or Zn /CaCl2, hydroxylamine is formed.
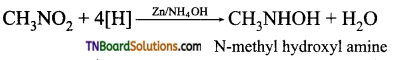
Question 9.
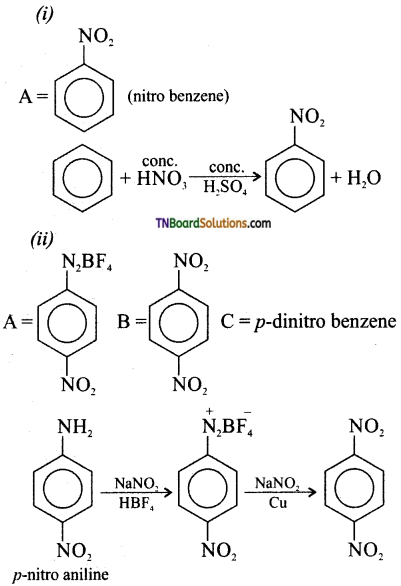
Complete the following equation. Identify A, B, and C.
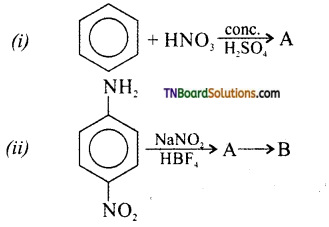
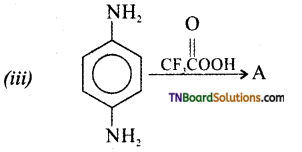
Answer:
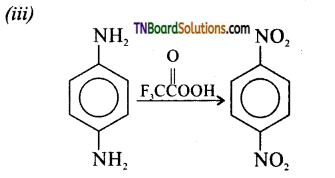
Question 10.
How does ethyl nitrite react with (i) Sn / HCl and (ii) HCl / H2O.
Answer:
![]()
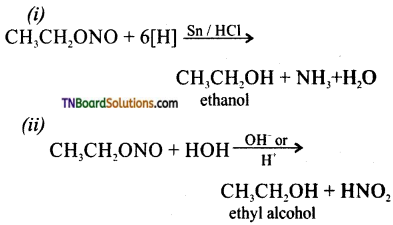
Question 11.
What is Nef carbonyl synthesis? Give equation.
Answer:
The formation of an aldehyde by the alkaline hydrolysis of a nitro alkane is known as Nef carbonyl synthesis.
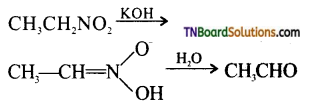
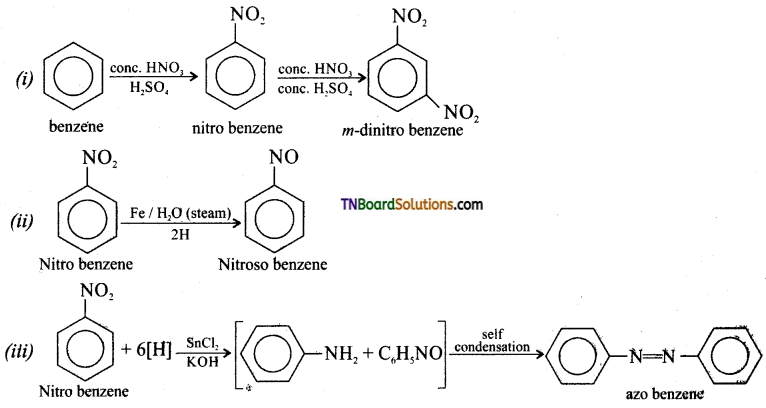
Question 12.
How are the following conversion made?
(i) Benzene to mcta dinitro benzene, (ii) Nitro benzene to nitroso benzene, (iii) Nitro benzene to azo benzene, (iv) Nitro benzene to hydrazo benzene.
Answer:
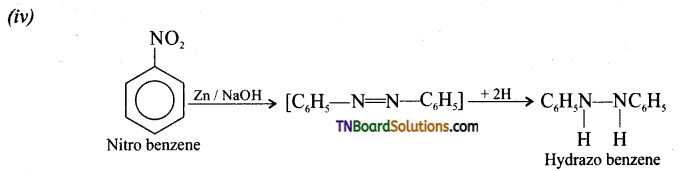
Question 13.
Identify the reagents used in the following conversions. Write complete equations.
(i) nitrobenzene to aniline
(ii) metadinitrobenzene to metanitroaniline
(iii) nitrobenzene to 3-nitrobenzene sulphonic acid.
Answer:
(i) Any reducing agent: Ni (or) Pt or LiAlH4

(ii) Ammonium poly sulphide
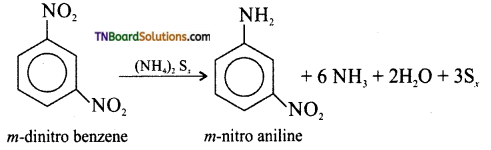
(iii) Conc. H2SO4
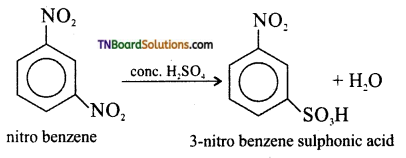
![]()
Question 14.
Explain why nitro group in nitro benzene is meta directing.
Answer:
The structure of nitro benzene is a resonance hybrid of the following cannonical structures.
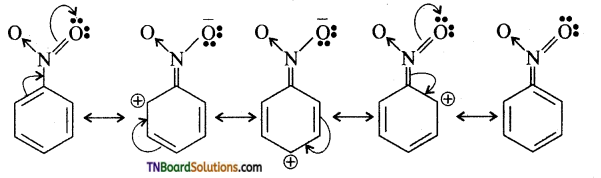
As a result of electron withdrawing nature of the NO2 group, the electron density at ortho and para position deefeases compafed to meta position i.e., the meta position is relatively electron rich compound to ortho and para positions. Hence the electrophile attacks the meta position.
Question 15.
How will you effect the following conversions?
(i) Benzene to m-chloro nitro benzene,
(ii) Benzene to 1, 2, 3 tri nitro benzene
(iii) m-chloro nitro benzenetto m-chloro aniline,
(iv) 1, 3, di nitrobenzene to 3- nitro aniline.
Answer:
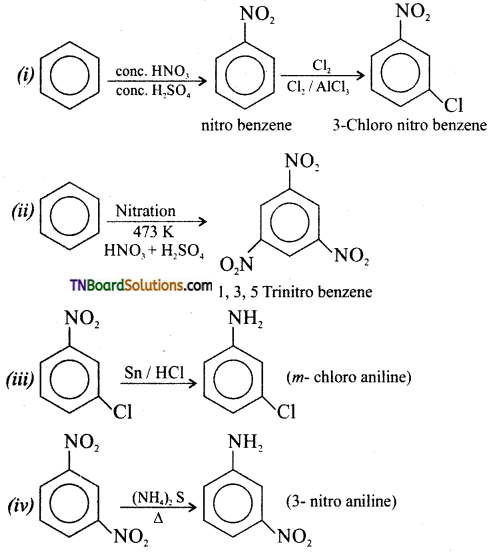
Question 16.
Give the IUPAC names of the following:
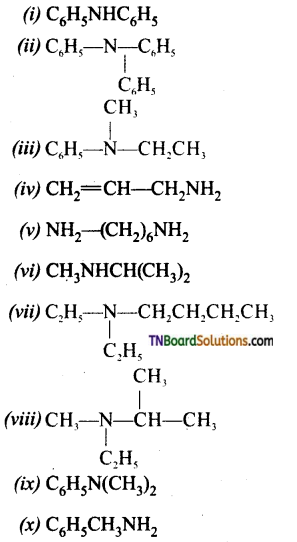
Answer:
(i) N -phenyl benzenamine
(ii) N, N -diphenyl benzenamine
(iii) N -methyl-N-phenylethanamine
(iv) prop -2- en-1-amine
(v) hexane-1, 6-diamine
(vi) N -methyl-propan-1-amine
(vii) N, N -diethyl-butan-1-amine
(viii) N -ethyl- N-methyl propan-2-amine
(ix) N, N -dimethyl-benzenamine
(x) phenyl-methanamine
![]()
Question 17.
Write the structures of the chain isomers of Butan-1-amine.
Answer:
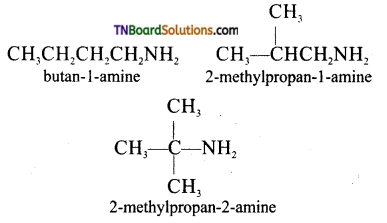
Question 18.
Explain ‘metamerism’ with a suitable example of amines.
Answer:
Aliphatic amines having the same molecular formula but different alkyl groups on either side of the nitrogen atoms show metamerism,
eg: CH3CH2NHCH2CH3 (diethyl amine) and CH3NHCH2CH2CH3 (methyl-n-propyl amine)
CH3NHCH(CH32)2 (Isopropyl methylamine) are metamers.
Question 19.
What are all the possible isomers of an amine having the molecular formula C3H9N and C4H11N.
Answer:
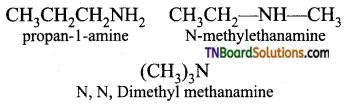
These amines exhibit functional isomerism.
Amine (C4H11N): CH3CH2CH2CH2NH2 (Butan-1-amine) exhibit chain as well as position isomerism.
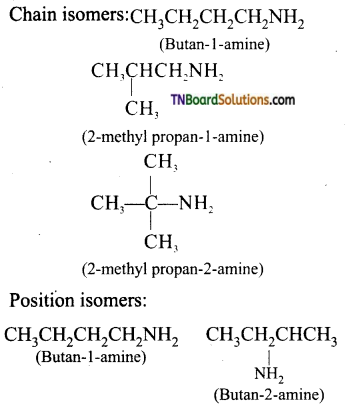
Question 20.
Write the structure of the following:
(i) 4N-dimethyl pentan-2-amine
(ii) 2 (N, N-dimethyl) butanamine
(iii) 2-aminoethanol
(iv) 4-aminobutanoicacid
(v) N-methyl-2-nitro pentanamine
(vi) prop-2-en-l -amine.
(vii) N-ethyl-N-methylbenzenamine
(viii) 3-methylbenzenamine
(ix) 2-methoxybenzenamine
(x) N-phenylbenzenamine
Answer:
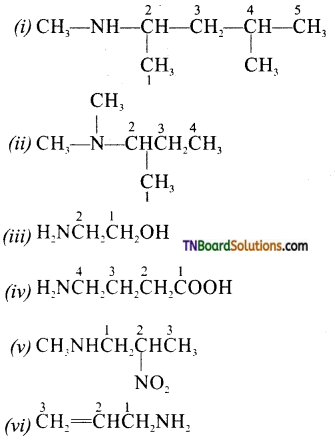
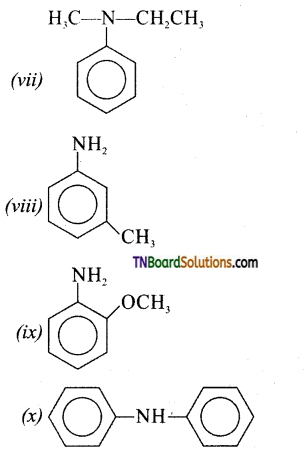
![]()
Question 21.
How is ethanamine prepared from (i) nitroethane, (ii) ethanenitrile, (iii) acetamide, (iv) ethyl bromide?
Answer:
(i) Reduction of nitroethane using H2 in the presence of Ni or Sn/HCl or Pd/H2.
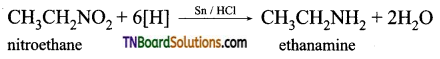
(ii) Reduction of ethanenitrile using sodium amalgam in ethanol.
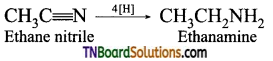
(iii) Reduction of acetamide using LiAlH4.

(iv) By Gabriel’s phthalimide synthesis.
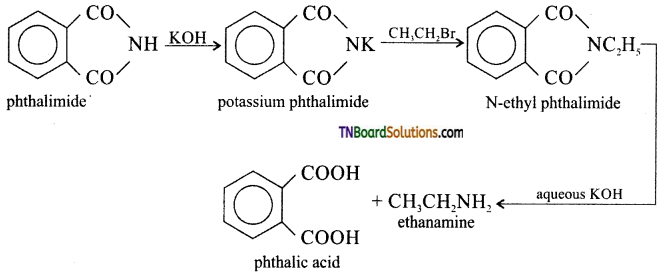
Question 22.
Identify the products:
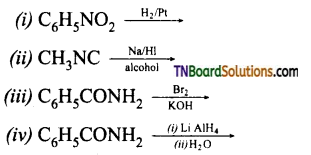
Answer:
(i) C6H5NH2 (aniline)
(ii) CH3NHCH3 (N-methyl methanamine)
(iii) C6H5NH2 (aniline)
(iv) C6H5CH2NH2 (benzyl amine)
Question 23.
Using sodium azide (NaN3) convert methyl bromide to methylamine.
Answer:

Question 24.
How will you prepare aniline from (i) chlorobenzene and (ii) phenol?
Answer:
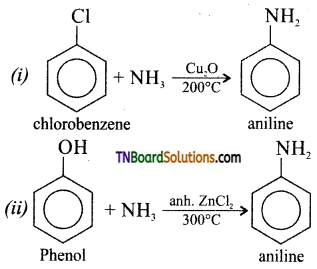
Question 25.
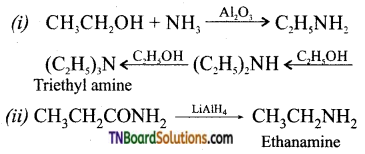
What happens when
(i) Vapours of ethanol and ammonia are passed over alumina.
(ii) Ethanamide is treated with LiAlH4. Give equation.
Answer:
![]()
Question 26.
Explain Why?
(i) Amines have higher boiling points than hydrocarbons of comparable molecular mass.
(ii) Among isomeric amines 3° amines have the lowest melting point.
(iii) The boiling point of amines are lower than those of alcohols and acids of comparable molecular mass.
(iv) Aliphatic amines with maximum six carbon atoms are soluble in water to some extent, while aromatic amines are insoluble in water.
Answer:
(i) This is due to the reason that amines being polar form inter molecular hydrogen bonding (except tertiary amines which do not have a H atom linked to N atom) and exist as associated molecules.
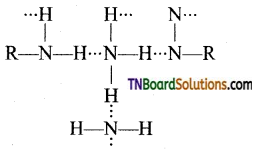
(ii) The degree of association depends on the extent of hydrogen bonding. Since 1° amines have two, 2° amines have one while 3° amines have no hydrogen linked to nitrogen. Therefore, among isomeric amine 1° amines have highest while 3° amine have the lowest boiling points.
(iii) The electronegativity of nitrogen is lower than oxygen, amines form weaker H—bonding compared to alcohol and carboxylic acids.i.e., The extent of association is less compared to that of alcohols or carboxylic acids. Hence amines will have lower boiling point compared to alcohol or carboxylic acid of comparable molecular mass.
(iv) All the three classes of amines (1°, 2° and 3°) form H bonds with water as a result lower aliphatic amines are soluble in water. As the size of the alkyl group increases (with increase in molecular mass), the solubility decreases due to a corresponding increase in hydrocarbon part of the molecule. The borderline solubility is reached with amines of about six carbon atoms in the molecule. Aromatic amines, on the other hand are insoluble in water. This is due to larger hydrocarbon part which tend to retard the formation of H-bonds.
Question 27.
Account for the fact that among ethyl amines, the decreasing order of basicity in aqueous solution is (CH3CH2)2NH > (CH3CH2)3N > CH3CH2NH2.
Answer:
The basicity of an amine in aqueous solution primarily depends upon the stability of the ammonium cation or the conjugate acid formed by accepting a proton from water. The stability of the ammonium cation in turn depends on the combination of the following factors.
(i) +1 effect of alkyl groups.
(ii) Extent of hydrogen bonding with water molecules.
(iii) Steric effects of the alkyl groups.
In the case of alkyl groups bigger than ‘CH3’ group i.e., ethyl, propyl, etc there will be steric hindrance to hydrogen bonding. As a result, stability due to +1 effect predominates over the stability due to H—bonding and hence 3° amines because more basic than 1° amines. In all overall decreasing basic strength of ethyl amine follows the sequence (CH3CH2)2NH > (CH3CH2)3N > CH3CH2NH2 > NH3.
![]()
Question 28.
How does nitrous acid (a mixture of sodium nitrite and dil. HCl) react with (i) CH3NH2 (ii) (CH3)2NH and (iii) (CH3)3N? Give equations.
Answer:
Aliphatic primary amines react with nitrous acid give an alcohol and nitrogen gas is produced. Methyl amine is an exception.
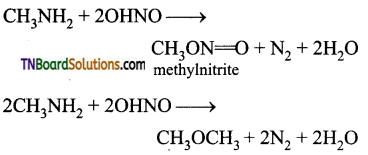
Secondary amines will form nitroso alkyl amine.
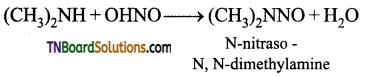
Tertiary amines react with nitrous acid and form nitrites.
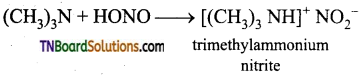
Question 29.
How does nitrous acid react with the following? Give equations.
(i) ethylamine, (ii) di-ethylamine, (iii) triethyl amine, (iv) aniline, (v) N-methyl aniline, (vi) N, N- dimethylaniline.
Answer:
(i) Ethylamine reacts with nitrous acid to give ethyl diazonium chloride, which is unstable and it is converted to ethanol by liberating N2.
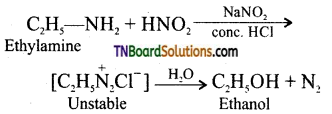
(ii) A yellow dye of N-nitrosodiethylamine is formed.

(iii) Triethylammoniumnitrate which is soluble in water formed.

(iv) Aniline reacts with nitrous acid at low temperature (273 – 278 K) to give benzene diazonium chloride which is stable for a short time and slowly decomposes even at low temperatures.
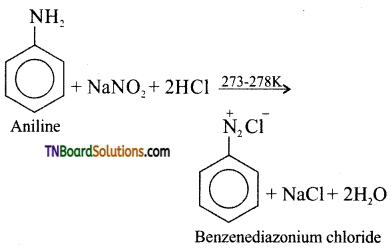
This reaction is known as diazotisation.
(v)
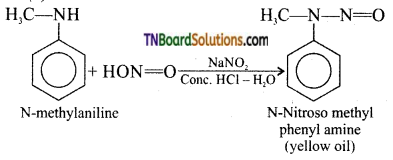
This reaction is known as Libermann’s nitroso test.
(vi) Aromatic tertiary amine reacts with nitrous acid at 273K to give p-nitroso compound.
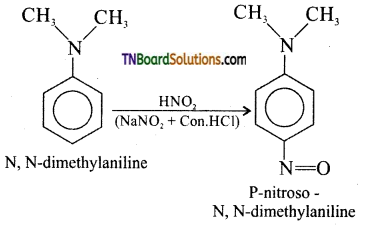
Question 30.
Explain why amino group is ortho para directing in electrophilic substitution reactions.
Answer:
The NH2 group is a strong activating group. In aniline the NH2 is directly attached to the benzene ring, the lone pair of electrons on the nitrogen is in conjugation with benzene ring which increases the electron density at ortho and para position, thereby facilitating the electrophilic attack at ortho and para.
![]()
Question 31.
Aniline gives 2, 4, 6 tribromo aniline when treated with bromine water, but not a mono bromo aniline. Explain why?
Answer:
Aniline is a more powerful activating group. The lone pair of electrons enters into conjugation with the benzene ring, as a result both ortho and para position become rich in electron density. At the same time Br+ is also a strong electrophile. Hence, all the three positions are attacked by the electrophile.
Question 32.
Why is it necessary to acetylate aniline to get a mono bromo aniline?
Answer:
Acetylation of aniline reduces the activity of the amino group. The activity of the acetylated amine is reduced because the lone pair of electron on nitrogen is delocalised by the neighbouring carbonyl group by resonance. Hence, it is not easily available for conjugation.
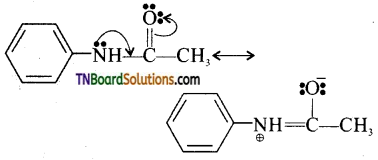
The acetyl amino group is less activating than amino group.
Question 33.
Explain why direct nitration of aniline gives a mixture of ortho, meta and para isomers.
Answer:
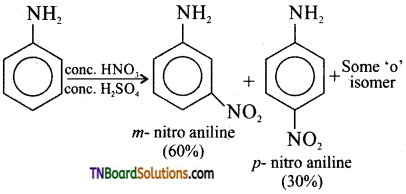
Nitration of aniline involves acidic medium. So protonation of aniline takes place forming mainly m-nitro aniline. Hence, direct nitration of aniline forms the following products.
Question 34.
How will you convert aniline?
(i) to p-bromo aniline
(ii) to 2,4,6 tribromo aniline
(iii) to para nitro aniline
(iv) to benzene diazonium chloride
(v) to sulphanilic acid
Answer:
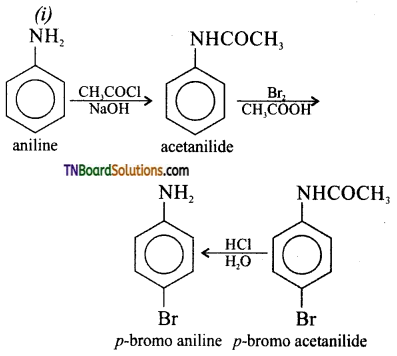
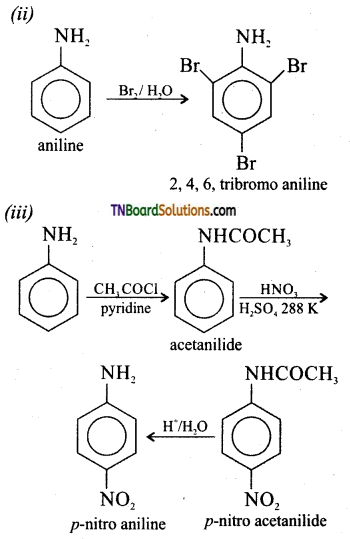
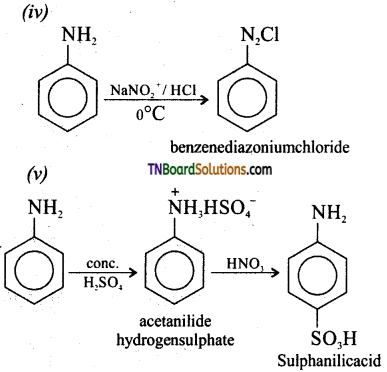
![]()
Question 35.
What is zwitter ion? Explain with an example.
Answer:
Zwitter ion is a compound in which both acidic and basic groups are present in the same molecule, eg: sulphanilic acid. It has an acidic (SO2OH) and a basic group (NH2). Thus a proton from the acid leaves and protonates the basic group. As a result it exists as a dipolar ion.

Question 36.
How will you distinguish between primary, secondary and tertiary amine?
Answer:
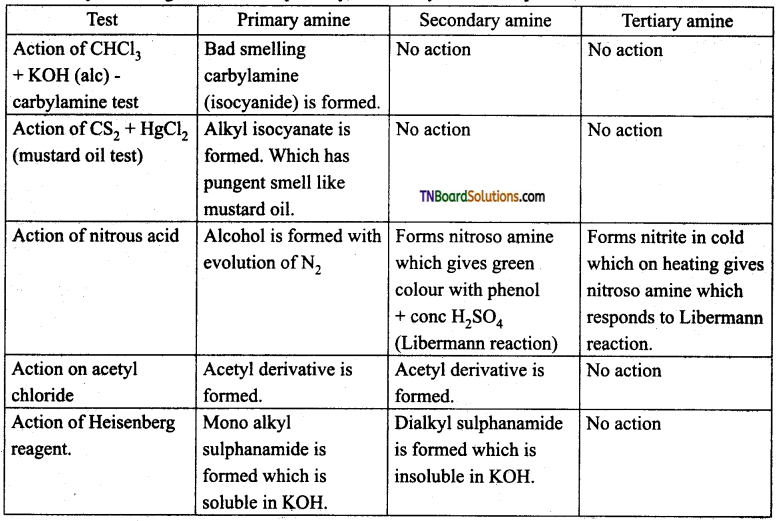
Heisenberg reagent: Benzene sulphoanyl chloride in the presence of excess aqueous KOH solution.
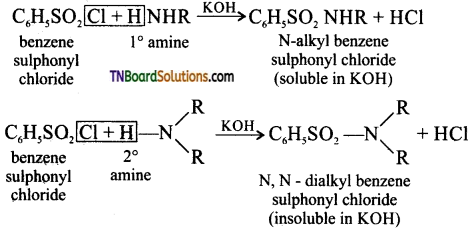
Question 37.
Complete the following reactions:
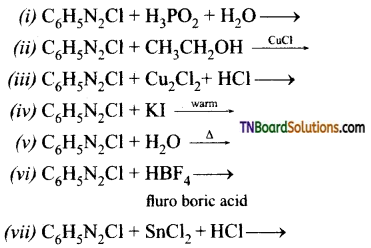
Answer:
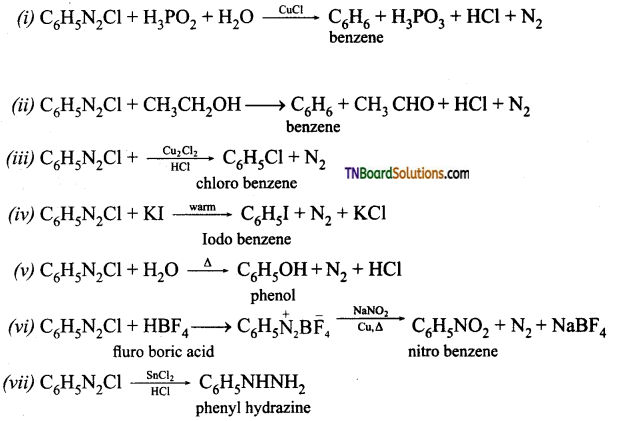
![]()
Question 38.
Give examples for (i) Sandmeyer’s reaction (ii) Gattermann reaction.
Answer:
(i) Sandmeyer’s reaction: Benzene diazonium chloride solution on reaction with cuprous chloride in hydrochloric acid (CuCl / HCl) or cuprous bromide in hydrochloric acid (CuBr / HCl) gives corresponding chloro benzene or bromo benzene. This reaction is known as Sandmeyer’s reaction. Aryl cyanides can also be prepared by this method.
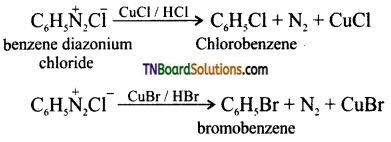
(ii) Gattermann reaction: This reaction involves the treatment of benzene diazonium chloride with Cu / HCl or Cu / HBr respectively instead of cuprous halide and hydrogen halide.
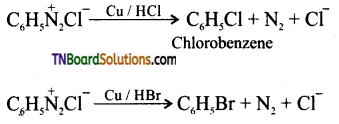
Question 39.
How does the following reagents react with benzene diazonium chloride? Give equations.
(i) HBF4 and the product formed is heated.
(ii) HBF4 and die product formed is heated with an aqueous solution of sodium nitrite in the presence of copper.
(iii) HBF4 and the product is heated with CH3COOH.
(iv) Cuprous cyanide in the presence of KCN.
Answer:
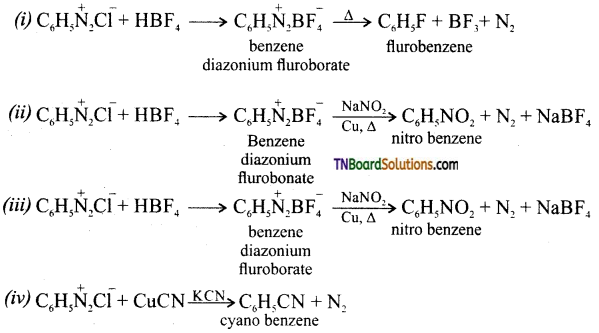
Question 40.
Accomplish die following conversions.
(i) Nitrobenzene to benzene,
(ii) 4-Nitro aniline to 1, 2, 3 -tribromo benzene,
(iii) p-toludine to 2-bromo-4-methyl aniline,
(iv) m-nitro aniline to m-chloroaniline,
(v) p-nitroaniline to p-iodomtro benzene,
(vi) benzyl chloride to 2-phenyl ethanamine.
Answer:
(i) Nitrobenzene to benzene:
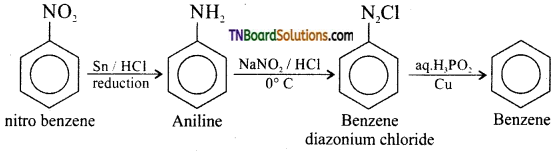
(ii) 4 -nitro aniline to 1, 2, 3 – tribromo benzene.
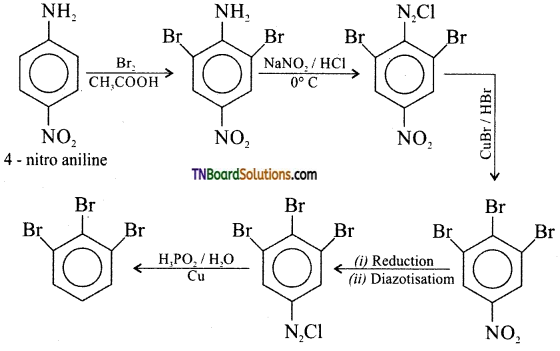
(iii) p-toludine to 2-bromo 4-methyl aniline.
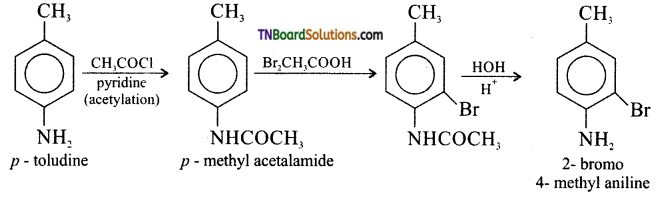
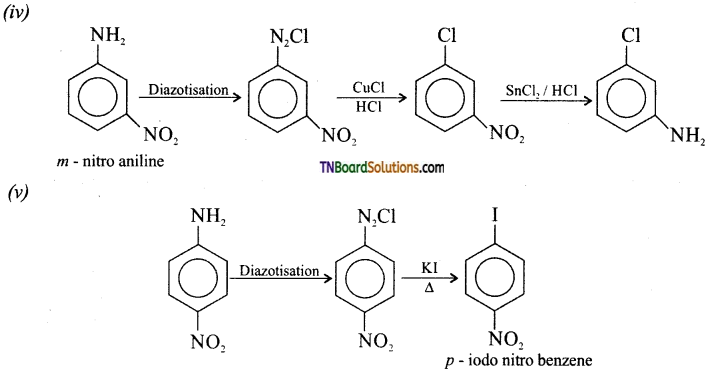
(vi) Benzyl chloride to 2- phenyl ethylamine.
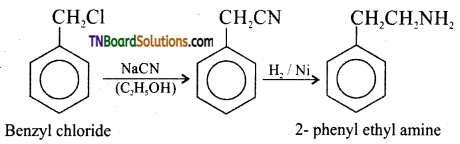
![]()
Question 41.
Give equations for the following reactions.
(i) Ethyl bromide is treated with KCN
(ii) Heating acetamide with P2O5
(iii) Heating acetaldoxime with P2O5
(iv) Treating methyl magnesium bromide with cyanogen chloride.
Answer:
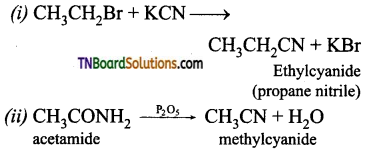
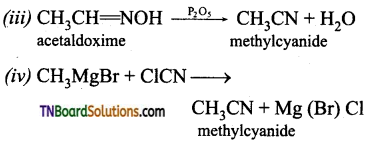
Question 42.
Write the IUPAC names of the following:
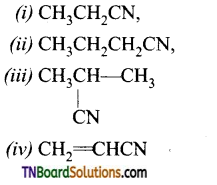
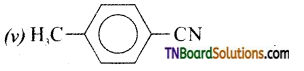
Answer:
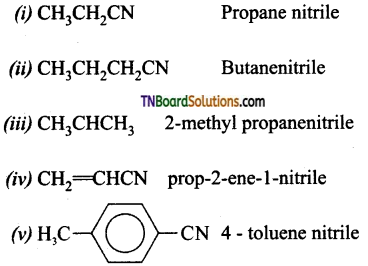
Question 43.
What is the reducing agent used in the following reduction reactions? Give equations.
(i) Ethanenitrile to ethanamine
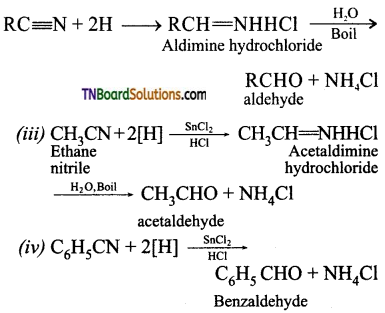
(ii) Benzonitrile to benzylamine
(iii) Ethane nitrile to Acetaldimine hydrochloride which on hydrolysis gives acetaldehyde.
Answer:
(a) Complete reduction or strong reduction when reduced with H2 in the presence of Pt or Ni as catalyst or by using LiAlH4, or sodium and alcohol alkyl or aryl cyanides yield primary amines.
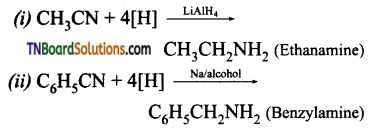
This specific type of reduction of alkyl or aryl cyanides using LiAlH4 or Na/ alcohol is named as mendices reduction.
(b) Partial reduction or mild reduction: When SnCl2 / HCl is used as a reducing agent at room temperature alkyl or aryl cyanides are reduced to amine hydrochloride which on subsequent hydrolysis gives aldehyde. This type of reduction is named as Stephen’s reduction.
SnCl2 + 2HCl → SnCl4 + 2 [H]
![]()
Question 44.
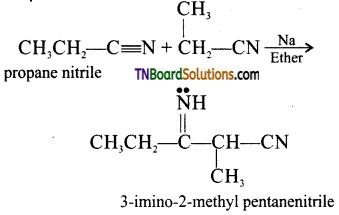
Give examples for (i) Thrope nitrile condensation and (ii) Levine and Hauser acetylation.
Answer:
(i) Thrope nitrile condensation: Self condensation of two molecules of alkyl nitrile (containing α—H atom) in the presence of sodium to form iminonitrile.
(ii) Levine and Hauser acetylation: The nitriles containing α-hydrogen also undergo condensation with esters in the presence of sodamide in ether to form ketonitriles. This reaction is known as “Levine and Hauser” acetylation. This reaction involves replacement of ethoxy (OC2H5) group by methylnitrile (-CH2CN) group and is called as cyanomethylation reaction.
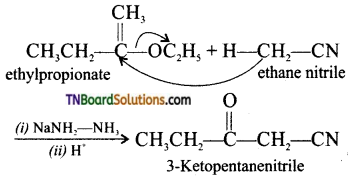
Question 45.
Write the electronic structure of alkyl cyanides and isocyanides.
Answer:
The electronic structure of alkyl cyanides is
![]()
The carbon atom of CN group is sp hybridised. CN group contain one σ and two pi bonds.
The electronic structure of isocyanides is
![]()
Structurally isocyanides are represented as a resonance hybrid of the following two forms.

The dipolar form (I) makes higher contribution.
Question 46.
Name the reagents used in the following reactions.
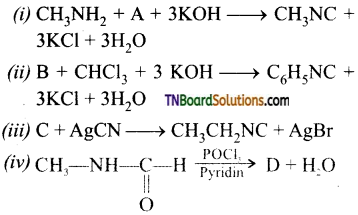
Answer:
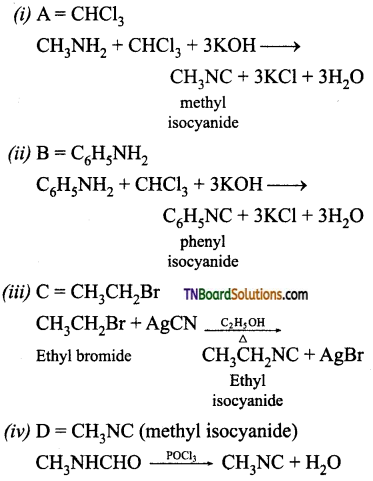
![]()
Question 47.
What happens when methyl isocyanide is:
(a) treated with dilute HCl.
(b) treated with sodium and alcohol
(c) heated at 250°C
(d) treated with sulphur in the presence of ozone.
Answer:
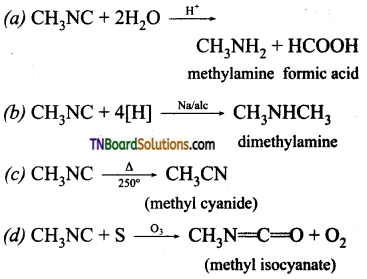
Question 48.
Mention the uses of (a) Nitro alkanes,
(b) Nitrobenzene,
(c) Cyanides and isocyanides
Answer:
(a) Nitroalkanes:
- Nitromethane is used as a fuel for cars.
- Chloropicrin (CCl3NO2) is used as an insecticide.
- Nitroethane is used as a fuel additive and precursor to explosive and they are good solvents for polymers, cellulose ester, synthetic rubber and dyes etc.,
- 4% solution of ethylnitrite in alcohol is known as sweet spirit of nitre and in used as diuretic.
(b) Nitrobenzene:
- Nitrobenzene is used to produce lubricating oils in motors and machinery.
- It is used in the manufacture of dyes, drugs, pesticides, synthetic rubber, aniline and explosives like TNT, TNB.
(c) Cyanides and isocyanides;
- Alkyl cyanides are important intermediates in the organic synthesis of larger number of compounds like acids, amides, esters, amines etc.
- Nitriles are used in textile industry in the manufacture of nitrile rubber and also as a solvent particularly in perfume industry.
Question 49.
An organic compound (A) having the molecular formula C2H7N is treated with nitrous acid to give (B) of molecular formula C2H6O which answers the iodoform test. Identify A and B and explain the reactions.
Answer:
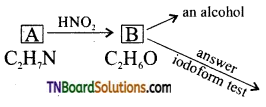
Since, ‘B’ is an alcohol formed from A by the action of nitrous acid, A should be a primary amine which contains ‘NH2’ group.
C2H5N—NH2=C2H5 i.e. The alkyl group is C2H5 and hence A is C2H5NH2 ethyl amine. B is ethyl alcohol and it is confirmed by the fact that it answer iodo form test.
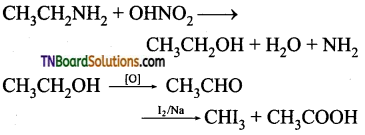
![]()
Question 50.
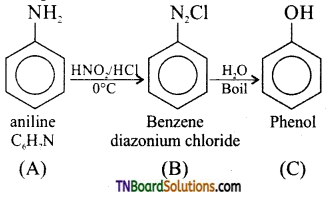
An organic compound (A) with molecular formula C6H7N gives (B) with HNO2 / HCl at 273K. The aqueous solution of (B) on heating gives compound (C) which gives violet colour with neutral FeCl3. Identify the compounds A, B and C. Write the equations.
Answer:
A = C6H5NH2 (aniline);
B = C6H2N2Cl (benzene diazonium chloride)
C = C6H5OH (phenol)
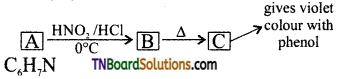
(i) Since ‘C’ gives a violet colouration with neutral FeCl3 it should be phenol.
(ii) Since phenol is obtained by boiling aqueous solution of (B), it should be benzene diazonium chloride.
(iii) Hence, A should be aniline.
Equation:
Choose the correct answer:
1.1, 2 dimethyl- 1-nitropropane. Choose the
incorrect statement about this compound.
(a) It is a primary nitro compound.
(b) It is also called nitroneopentane.
(c) The ‘NO2’ group is attached to a secondary carbon atom.
(d) It is an aliphatic compound.
Answer:
(c)
2. 1-nitrobutane and 2-methyl-1-nitro propane:
(a) chain isomers
(b) position isomers
(c) functional isomers
(d) metamers
Answer:
(a)
Hint:

![]()
3. Which of the following nitro compounds does not exhibit tautomerism?
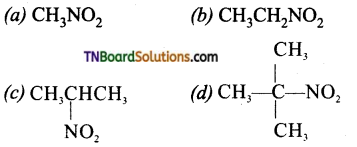
Answer:
(d)
Hint: Tertiary nitro alkanes do not exhibit tautomerism due to absence of a-H atoms.
4. The incorrect statement between nitroform and acidform of nitromethane is:
(a) nitroform is less acidic whereas aciform is more acidic.
(b) nitroform dissolves in NaOH slowly while aciform dissolves in NaOH instantly.
(c) Both decolorise FeCl3 solution
(d) They are tautomers
Answer:
(c)
Hint: Nitroform decolorises FeCl3 solution while aciform gives a reddish brown colour with FeCl3 solution.
5.
![]()
Identify A and B.
(a) A = CH3CH2NO2 ; B = CH3CH2NH2
(b) A- CH3NO2 ; B = CH3NH2
(c) A = CH3CHO ; B = CH3CH2OH
(d) A = CH3CH2NH2 ; B = CH3CH2OH
Answer:
(a)
6.
Identify A and B.
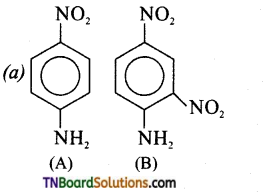
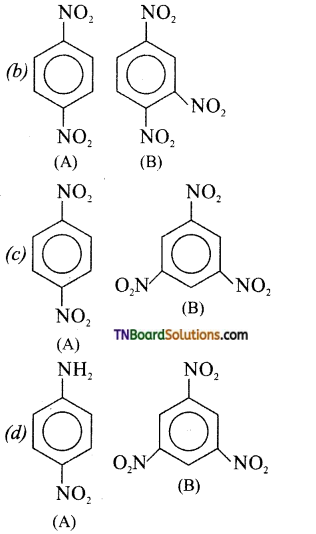
Answer:
(b)
![]()
7. The products obtained by the reduction of nitromethane in acid medium and neutral medium are:
(a) methylamine and N-methylhydroxylamine
(b) methylamine and ethanol
(c) N-methylhydroxylamine and methylamine
(d) N-methylhydroxylamine in both cases.
Answer:
(a)
Hint:
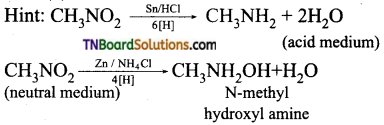
8. An amine is boiled with HCl and H2O. Which of the following will give acetone:
(a) CH3NH2
(b) (CH3)2CHNO2
(c) (CH3)3CNO2
(d) both (b) and (c)
Answer:
(b)
Hint: CH3NH2 gives CH3COOH (CH3)2
CHNO2 gives (CH3)2CO
(CH3)3 CNO2 no reaction
9. The correct IUPAC name for CH2=CH—CH2NH—CH3 is:
(a) Allylmethyl amine
(b) 2-amino-4-pentane
(c) 4 amino pent-l-ene
(d) N-methyl prop-2 en-1 amine
Answer:
(d)
10. Amongst the following the strongest base in aqueous medium is:
(a) CH3NH2
(b) NC—CH2NH2
(c) (CH3)2NH
(d) C6H5NH—CH3
Answer:
(c)
Hint: 2° amines are more basic than 1° amines, i. e., (CH3)2NH is more basic than CH3NH2. Due to -I effect of CN group NC—CH2NH2 is less basic than even CH3NH2. C6H5NHCH3 is less basic than both C6H5NH2 and (CH3)2NH due to delocalisation of lone pair of electrons on the N atom into the benzene ring.
11. In order to prepare a 1 ° amine from an alkyl halide with simultaneous addition of one CH2 group in the carbon chain, the reagent used a source of nitrogen is:
(a) Sodium amide, NaNH2
(b) Sodium azide, NaN3
(c) Potassium cyanide, KCN
(d) Potassium phthalimide C6H4(CO2) N–K–
Answer:
(c)
Hint: Cyanides on reduction gives 1: amines with a CH2 group

12. The correct increasing order of basic strength for the following compounds is:
(a) II < III < I
(b) III < I < II
(c) III < II < I
(d) II < I < III
Answer:
(b)
![]()
13. Best method for preparing primary amines from alkyl halides without changing the number of carbon atoms in the chain is:
(a) Hoffmann bromamide reaction
(b) Gabriel’s phthalimide synthesis
(c) Sandmeyer’s reaction
(d) Reaction with NH3
Answer:
(b)
14. Which of the following compounds is the weakest Bronsted base?
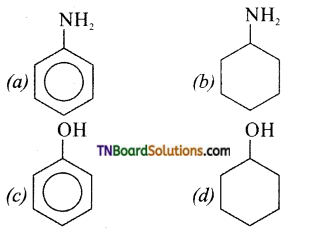
Answer:
(c)
Hint: Amines (a) and (b) have a stronger tendency to accept a proton and hence stronger Bronsted bases than phenol (c) and alcohol (d) since phenol is more acidic than alcohol, phenol (c) has the least tendency to accept a proton and hence the weakest Bronsted base.
15. Which of the following compounds cannot be prepared by Sandmeyer’s reaction?
(I) Chlorobenzene
(II) Bromobenzene
(III) Iodo benzene
(IV) Fluoro benzene
(a) (IV)
(b) (III)
(c) (I) and (II)
(d) (III) and (IV)
Answer:
(a)
![]()
16. The products of the following reaction are:
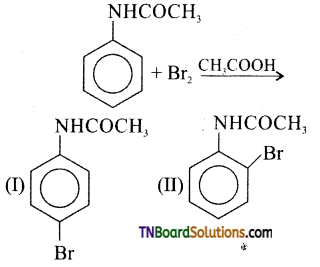
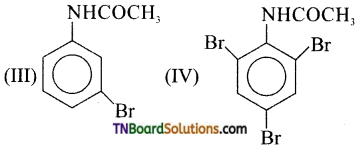
Answer:
(c)
Hint: —NHCOCH3 is an o/p directing group.
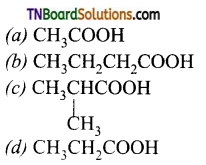
17. An organic compound ‘A’ on treatment with NH3 gives ‘B’ which on heating gives ‘C’. ‘C’ when treated with Br2 in the presence of KOH produces ethylamine. The compound ‘A’ is:
Answer:
(d)
Hint:
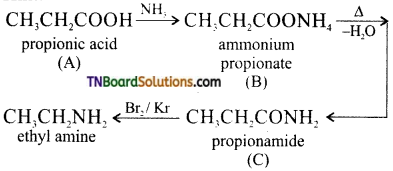
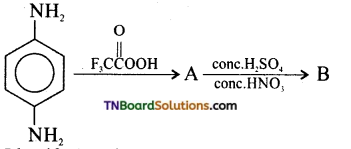
18. In a set of reactions, meta mono benzoic acid gave a product ‘D’. Identify D.
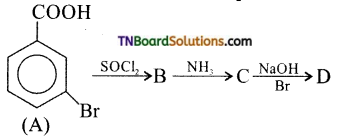
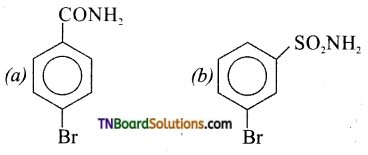
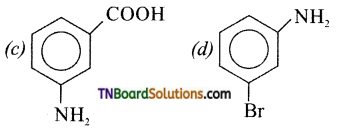
Answer:
(d)
Hint:
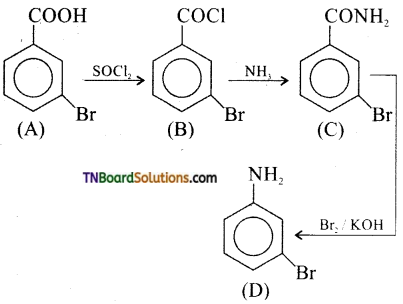
![]()
19.
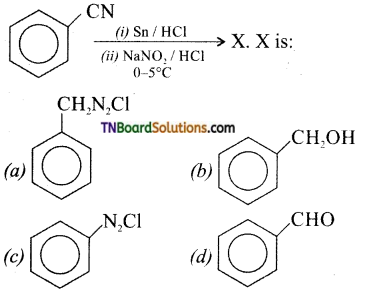
Answer:
(b)
Hint: Diazonium salts of benzylamine is not stable. It decomposes insitu, to form benzyl alcohol.
20. Predict the product:
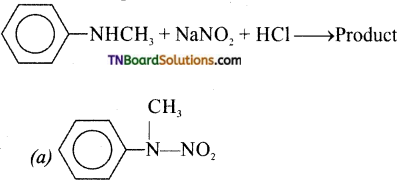
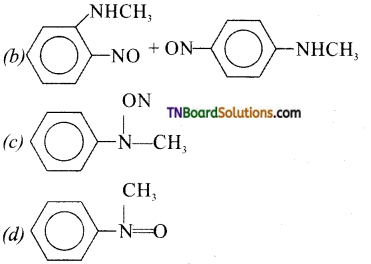
Answer:
(d)
21. Aniline in a set of following reactions yielded a coloured product Y. The structure of Y would be:
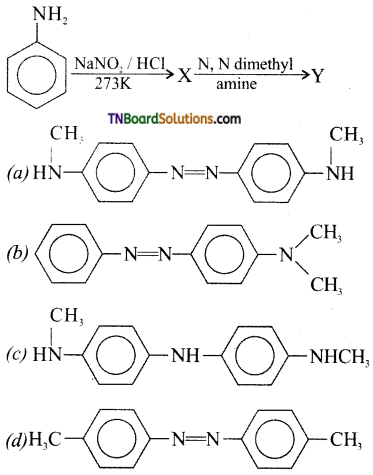
Answer:
(b)
Hint:
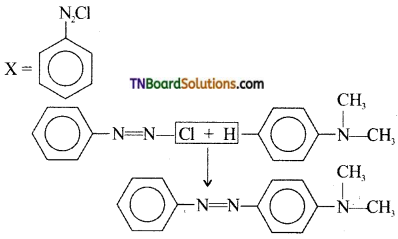
![]()
22. Butylamine (I), diethyl amine (II) and N, N diethyl amine, (III) have the same molar mass. The increasing order of their boiling points is:
(a) III < II < I
(b) I < II < III
(c) II < III < I
(d) III < I < II
Answer:
(a)
Hint: Extent of H-bonding increases as the number of H-atoms increases and hence boiling point increases in the order 3°<2°< 1°.
23. Assertion: Ammonolysis of alkyl halides is not a suitable method for the preparation of primary amines.
Reason: Ammonolysis of alkyl halides mainly produces 2° amines.
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
(b) Both assertion and reason are true and reason is not the correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(c)
24. Assertion: Gabriel phthalimide reaction can be used to prepare aryl and arylalkyl amines.
Reason: Aryl halides are as reactive as alkyl halides towards nucleophilic substitution reactions.
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
(b) Both assertion and reason are true and reason is not the correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(d)
Hint: Correct assertion: Gabriel phthalimide reaction can be used to prepare alkyl and aryl alkyl primary amines.
Correct reason: Alkyl and aralkyl halides undergo nucleophilic substitution reaction but aryl halides do not.
![]()
25. Assertion: Aniline does not undergo Friedel Craft’s reaction.
Reason: Friedel crafts reaction is an electrophilic substitution reaction.
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
(b) Both assertion and reason are true and reason is not the correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(b)
Hint: Correct explanation: AlCl3 forms a salt with aniline (C6H5NH2+ AlCl3–) which deactivates the benzene ring thereby preventing Friedel craft’s reaction.