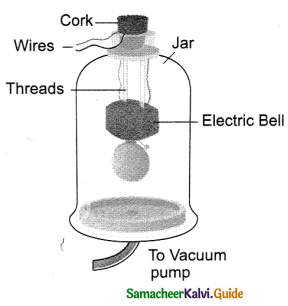
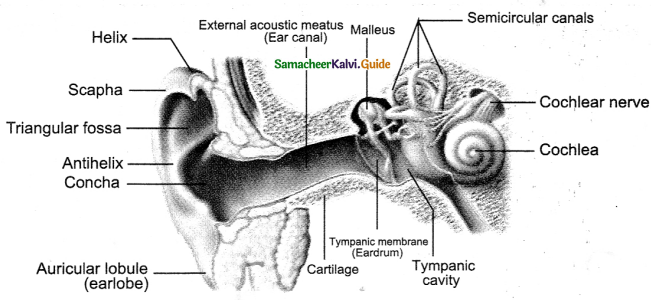
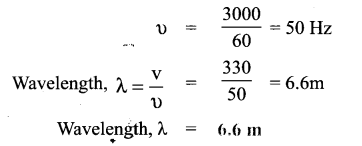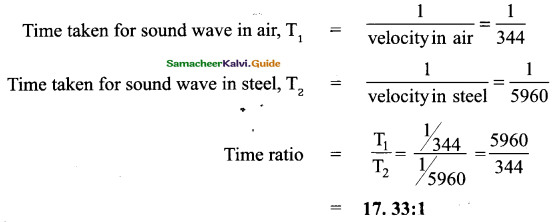Tamilnadu State Board New Syllabus Samacheer Kalvi 9th Science Guide Pdf Chapter 25 Computer – An Introduction Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 9th Science Solutions Chapter 25 Computer – An Introduction
9th Science Guide Computer – An Introduction Text Book Back Questions and Answers
![]()
I. Choose the correct answer.
Question 1.
………………. is an electronic device which stores data and information.
(a) Telescope
(b) Television
(c) Computer
(d) Radio
Answer:
(c) Computer
Question 2.
………………. belongs to the generation IV of the computer
(a) Microprocessor
(b) Artificial Intelligence
(c) Transistor
(d) Vaccum Tubes
Answer:
(a) Microprocessor
![]()
Question 3.
Data processing involves……………….steps.
(a) seven
(b) four
(c) six
(d) eight
Answer:
(c) six
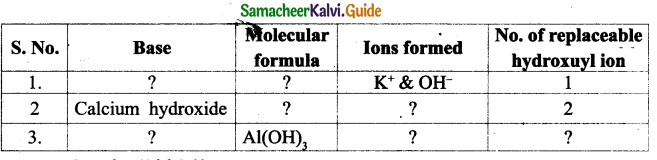
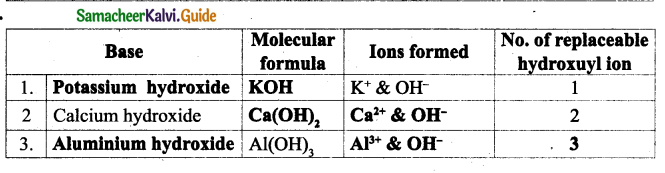
II. Match the following:
| III generation computer | Integrated circuit |
| Text, | Information |
| Transistor | Father of computer |
| Directly used | Data |
| Charles Babbage | II generation |
Answer:
| III generation computer | Integrated circuit |
| Text, number | Data |
| Transistor | II generation |
| Directly used | Information |
| Charles Babbage | Father of computer |
![]()
III. Answer briefly :
Question 1.
Define computer.
Answer:
A computer is an electronic device, which manipulates and stores data and information through commands or program codes.
Question 2.
Differentiate data and information.
Answer:
Data
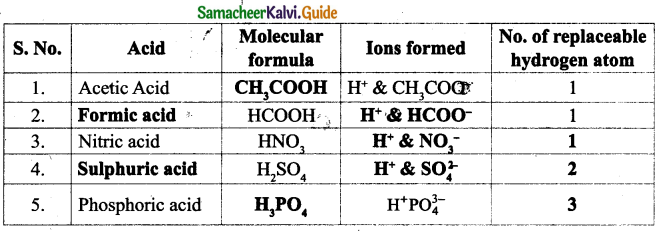
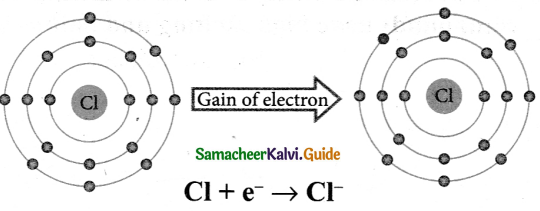
Data is the set of values of qualitative and quantitative variables. The data that is fed to the computer can be text, numbers, or statistics. These data stored in computer memory cannot be used directly. It has to be processed.
Information
The information we get or obtain or receive using the data from the computer can be used directly.
![]()
Question 3.
What is data processing?
Answer:
The data processing in a computer is collecting data and converting it into information according to our needs and requirements.
IV. Answer in detail :
Question 1.
What are the different steps involved in data processing?
Answer:
Data processing has six steps. They are,
- Data collection
- Data storage
- Data sorting
- The data processing
- Data analysis
- Data presentation and conclusions
![]()
Question 2.
List out the generations of computers.
Answer:
The history of computers has been classified into many stages. The main difference between the generations is the speed and efficiency of the computer. On the basis of performance and speed, the generations of the computer was categorised as follows.
Generations of Computer:
| Period | Generation | Digital devices |
| 1940-1956 | I Generation | Vacuum tubes |
| 1956-1963 | II Generation | Transistor |
| 1964-1971 | III Generation | Integrated circuits |
| 1972-2010 | IV Generation | Microprocessors |
| After 2010 | V Generation | Artificial Intelligence |
![]()
9th Science Guide Computer – An Introduction Additional Important Questions and Answers
I. Choose the correct answers :
Question 1.
The people of China used ……………….
(a) abacus
(b) transistor
(c) vacuum tubes
(d) microprocessors
Answer:
(a) abacus
![]()
Question 2.
ENIAC computer had approximately ………………. vacuum tubes.
(a) 16,000
(b) 14,000
(c) 18, 000
(d) 12,000
Answer:
(c) 18,000
Question 3.
ATM machine works with the help of …………… only.
(a) calculator
(b) mobile
(c) transistor
(d) computer
Answer:
(d) computer
Question 4.
How many generations of computers are there?
(a) 2
(b) 5
(c) 3
(d) 6
Answer:
(b) 5
![]()
Question 5.
………………. was used by the American Military.
(a) ENIAC
(b) Computer
(c) Transistor
(d) Calculator
Answer:
(a) ENIAC
Question 6.
The main difference between the generations is the ………… and …………….. of the computer.
(a) size, shape
(b) hardware, software
(c) speed, efficiency
(d) shape, performance
Answer:
(c) speed, efficiency
Question 7.
………….. is one of the steps in the data processing.
(a) Data management
(b) Data storage
(c) Data calculation
(d) Data transformation
Answer:
(b) Data storage
![]()
Question 8.
………………….. stored in computer memory cannot be used directly.
Information
a) Information
(b) Words
(c) Data
(d) Numbers
Answer:
(c) Data
Question 9.
Which of the following is the period of the first generation of computers?
(a) 1956- 1963
(b) 1964- 1971
(c) 1940- 1956
(d) 1972-2010
Answer:
(c) 1940 – 1956
![]()
Question 10.
(i) The computer operates by the exchange of commands between hardware and
software.
(ii) Lady Augusta Ada Lovelace gave the first programming to do arithmetic operations.
(a) Both the statements are Wrong
(b) Statement (i) is correct but’ statement (ii) is wrong
(c) Statement (i) is wrong but statement (ii) is correct
(d) Both the statements are correct
Answer:
(d) Both the statements are correct
![]()
II. Match the following :
| ATM | set of values |
| Lady Augusta Ada Lovelace | step in the data processing |
| Data analysis | withdraw money |
| Data | general-purpose computer |
| ENIAC | 1956 – 1963 |
| II Generation | 1972 – 2010 |
| IV Generation | First programmer |
Answer:
| ATM | withdraw money |
| Lady Augusta Ada Lovelace | First programmer |
| Data analysis | step in the data processing |
| Data | set of values |
| ENIAC | general-purpose computer |
| II Generation | 1956 – 1963 |
| IV Generation | 1972 – 2010 |
![]()
III. Answer briefly:
Question 1.
Define ENIAC.
Answer:
- ENIAC (Electronic Numerical Integrator And Computer) was designed in the year 1946 was equivalent to the size of a huge classroom.
- ENIAC had approximately 18,000 vacuum tubes.
- ENIAC, which was used by the American Military in 1946 to predict the trajectory of artillery shells, recognized as the world’s first general-purpose computer.
Question 2.
What is meant by hardware and software?
Answer:
- The computer operates by the exchange of commands between the hardware and software.
- Hardware can be touched and felt.
- The software cannot be touched and felt.
![]()
Question 3.
Name the fields where computers are used.
Answer:
The fields where computers are used are:
- Banks
- Hospitals
- Post offices
- Transport
- Market
- Media
- Defense sector
- Education and space research
IV. Answer in detail :
Question 1.
Explain the history of computers.
Answer:
- Around 2000 years ago, the people of China used Abacus.
- This was considered as the most basic model of a computer.
- Nineteenth century was considered as the birth of the computer when Charles Babbage designed the basic construction of a computer.
- ENLAC, which was used by the American Military in 1946 to predict the trajectory of artillery shells, recognized as the world’s first general-purpose computer.
- ENIAC had approximately 18,000 vacuum tubes.
- The size occupied by the ENIAC could be equivalent to a classroom.
- Lady Augusta Ada Lovelace was honored as the first programmer as she gave the first programming to do arithmetic operations.
![]()
Question 2.
Write the history of computers.
Answer:
- Around 2000 years ago, the people of China used Abacus. This was considered as the most basic model of a computer.
- Nineteenth-century was considered as the birth of the computer when Charles Babbage designed the basic construction of a computer.
- ENIAC, which was used by the American Military in 1946 to predict the trajectory of artillery shells was recognized as the world’s first general-purpose computer.
- Lady Augusta Ada Lovelace was honored as the first programmer as she gave the first programming to do arithmetic operations.
Question 3.
Explain in detail about data and data processing.
Answer:
Data:
- Data is the set of values of qualitative and quantitative variables.
- The data that is fed to the computer can be text, numbers or statistics.
- These data stored in computer memory cannot be used directly.
- It has to be processed.
![]()
Data Processing:
The data processing in a computer is collecting data and converting it into information according to our needs and requirements.
Steps in data processing:
Data processing has six steps. They are:
- Data collection
- Data storage
- Data sorting
- Data processing
- Data analysis
- Data presentation and conclusions
![]()
Question 4.
What are the different generations of computers?
Answer:
- The main difference between the generations is the speed and efficiency of the computer.
- On the basis of performance and speed, the generations of the computer were categorised.
Generations of Computer:
| Period | Generation | Digital devices |
| 1940-1956 | I Generation | Vacuum tubes |
| 1956-1963 | II Generation | Transistor |
| 1964-1971 | III Generation | Integrated circuits |
| 1972-2010 | IV Generation | Microprocessors |
| After 2010 | V Generation | Artificial Intelligence |



 s
s