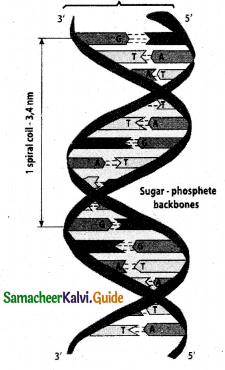
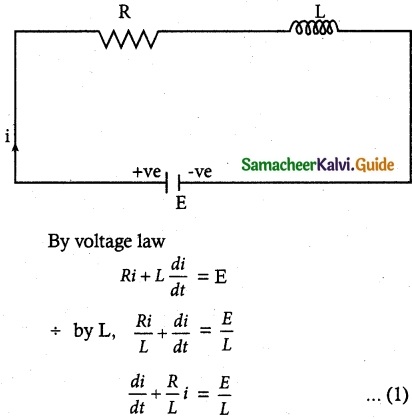
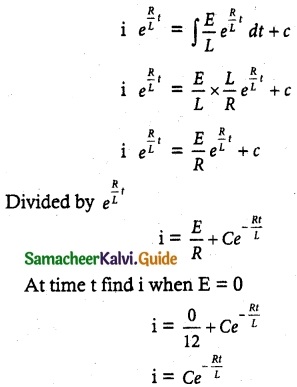
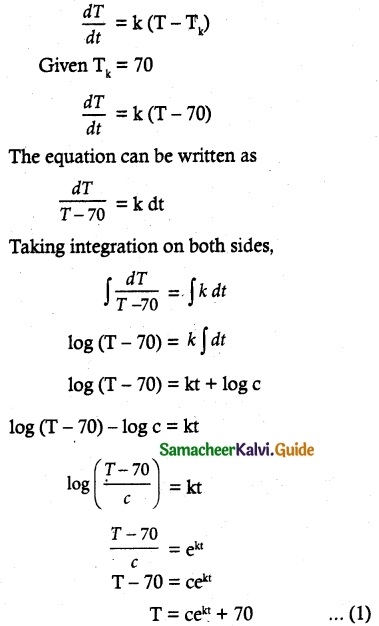
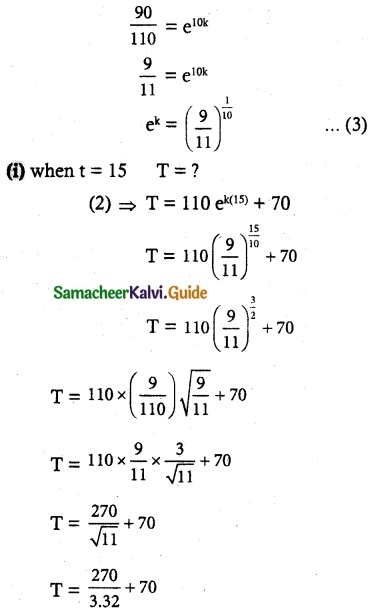
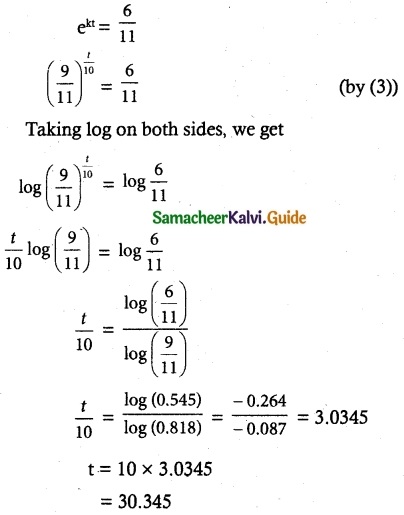
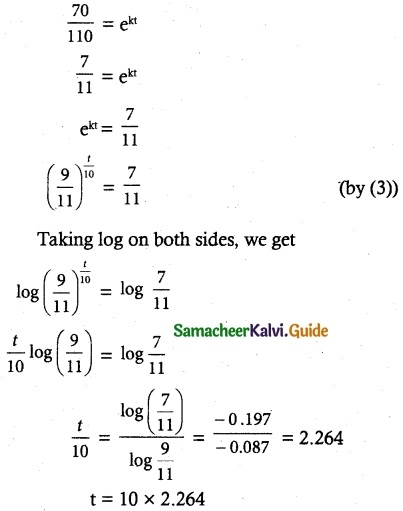
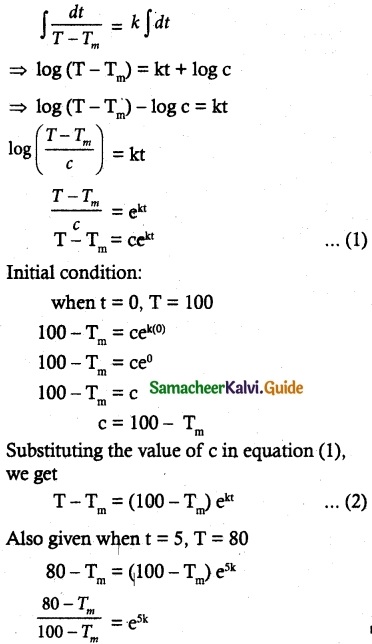
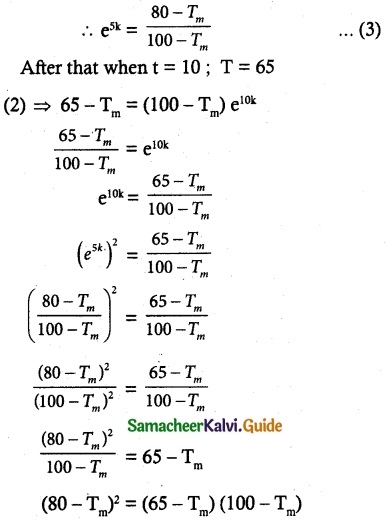
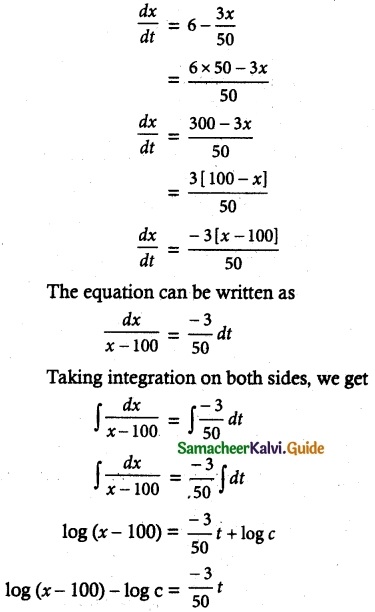
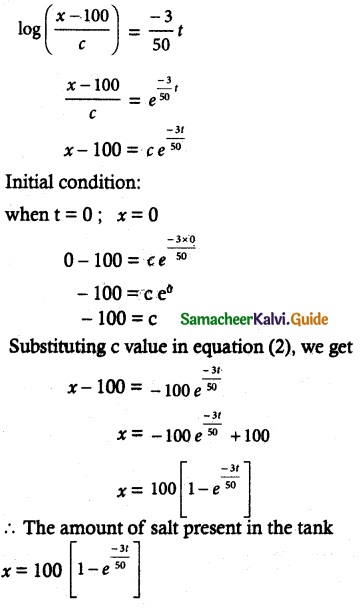
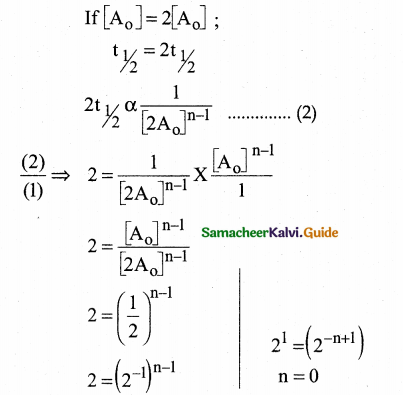
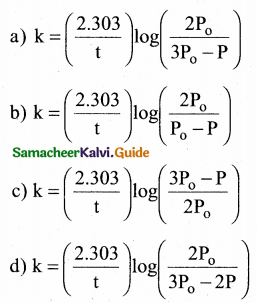
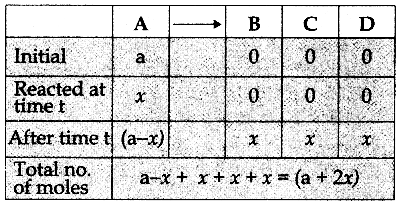
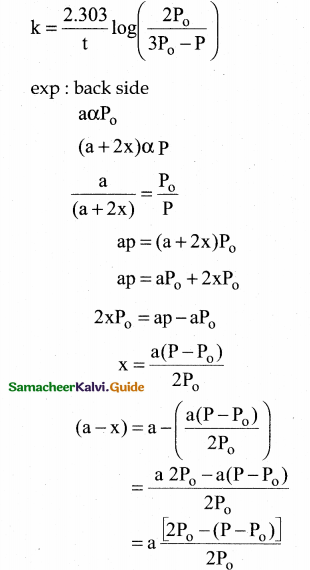
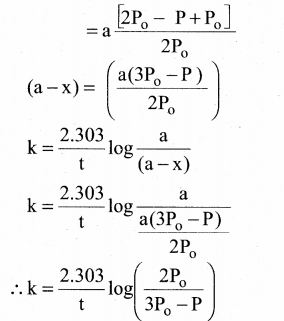
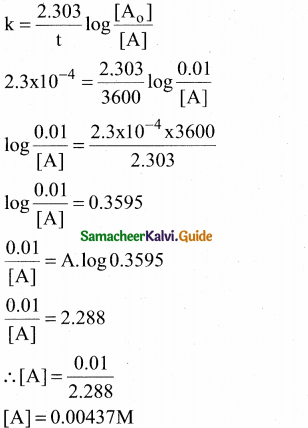
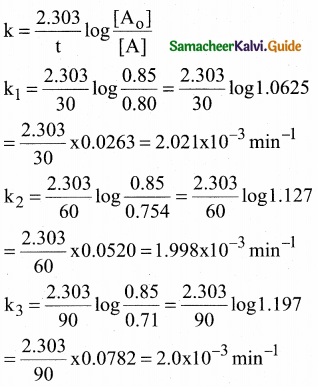
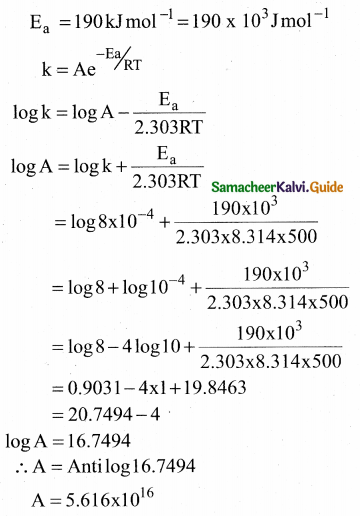
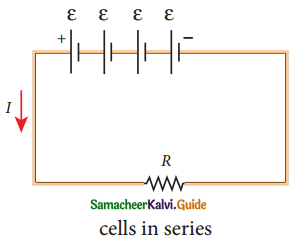
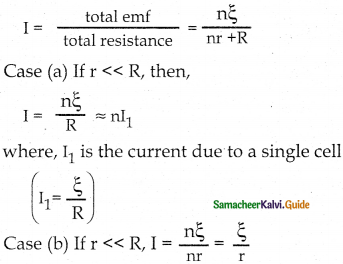
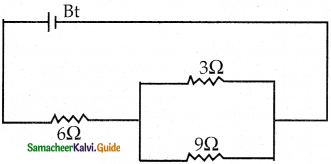
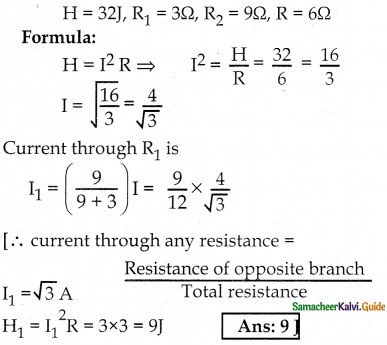
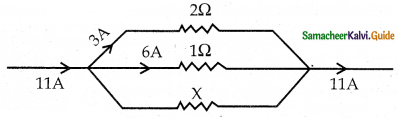
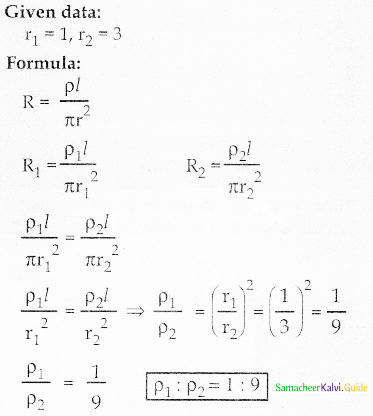
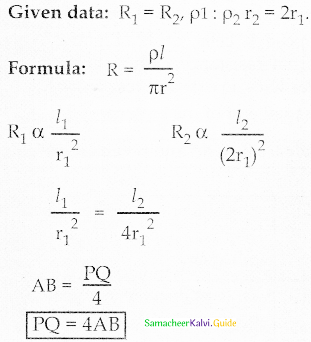
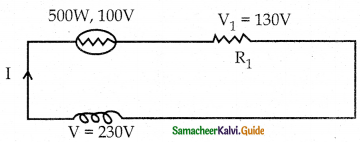
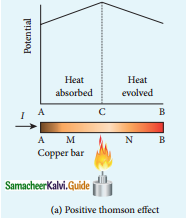
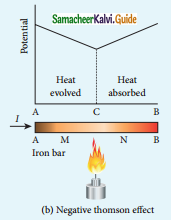
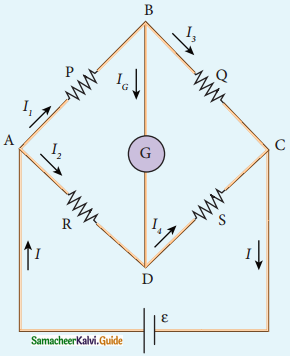
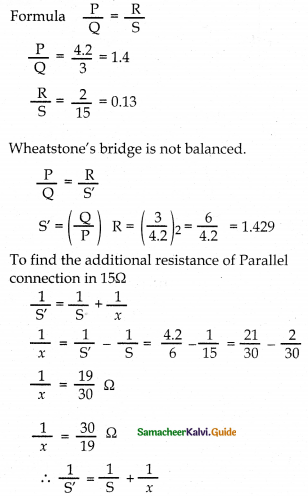
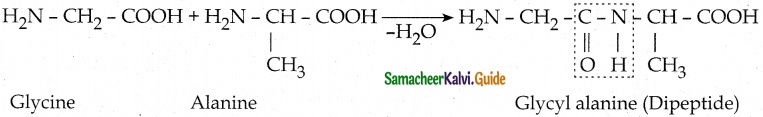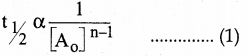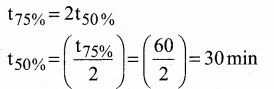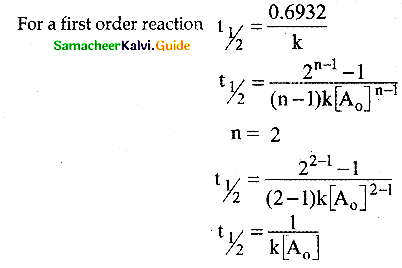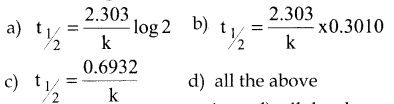Tamilnadu State Board New Syllabus Samacheer Kalvi 12th Commerce Guide Pdf Chapter 9 Human Resource Management Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 12th Commerce Solutions Chapter 9 Human Resource Management
12th Commerce Guide Human Resource Management Text Book Back Questions and Answers
I. Choose The Correct Answer.
Question. 1
Human resource is a …………. asset.
a) Tangible
b) Intangible
c) Fixed
d) Current
Answer:
b) Intangible
Question 2.
Human Resource management is both ………………….. and ………………..
a) Science and art
b) Theory and practice
c) History and Geography
d) None of the above
Answer:
a) Science and art
![]()
Question 3.
Planning is a …………. Function.
a) selective
b) pervasive
c) both a and b
d) none of the above
Answer:
b) pervasive
Question 4.
Human resource management determines the ……………………….. relationship.
a) internal, external
b) employer, employee
c) Owner, Servant
d) Principle, Agent
Answer:
b) employer, employee
Question 5.
Labour turnover is the rate at which employees ………….. the organisation.
a) enter
b)leave
c) salary
d)None of the above
Answer:
b) leave
![]()
II. Very Short Answer Questions.
Question 1.
Give the meaning of Human Resource.
Answer:
In an organisation, the human resource is the employees who are inevitable for the survival and success of the enterprise.
Question 2.
What is Human Resource Management?
Answer:
The Branch of Management that deals with managing resource is known as “Human Resource Management (HRM)
![]()
Question 3.
State two features of HRM.
Answer:
Features of Human Resource Management.
Universally relevant: Human Resource Management has universal relevance.
Goal-oriented: The accomplishment of organisational goals is made possible through the best utilisation of human resources in an organisation.
Question 4.
Mention two characteristics of Human Resource. [OWE]
Answer:
- HR is Only factor of production that lives.
- HR can be Work as a team.
- HR – Exhibits innovations and creativity.
![]()
Question 5.
What are the managerial functions of HRM.
Answer:
The functions of human resource management may be classified as under: I Managerial function – Planning, Organising, Directing, Controlling.
II Operative function – Procurement, Development, Compensation, Retention, Integration, Maintenance.
III. Short Answer Questions.
Question 1.
Define the term Human Resource Management.
Answer:
HRM – Definition:
“HRM as that part of management process which is primarily concerned with the human constituents of an organisation”. E.F.L. BRECH
![]()
Question 2.
What are the characteristics of Human resources? [OWE] [MOM]
Answer:
- Human resource exhibits innovation and creativity.
- Human resources alone can think, act, analyse and interpret.
- Human resources are emotional beings.
- Human resources can be motivated either financially or non-financially.
- The behaviour of human resources is unpredictable.
- Over the years human resources gain value and appreciation.
- Human resources are movable.
- Human resources can work as a team.
Question 3.
What is the significance of Human resource?
Answer:
- HR is possible only through human relations.
- HR managers all other factors of production.
- HR can be utilised at all levels of management.
- HR is helpful to utilise all other resources.
- HR helps in industrial relation.
- HR well protected by legal frame works.
![]()
Question 4.
State the functions of Human Resource Management.
Answer:
The functions of human resource management may be classified as under: I Managerial function – Planning, Organising, Directing, Controlling.
II Operative function – Procurement, Development, Compensation, Retention, Integration, Maintenance.
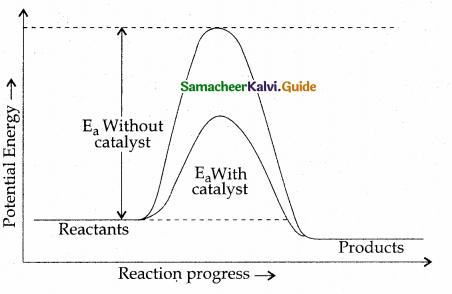
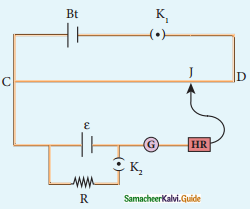
- Planning: Planning is deciding in advance what to do, how to do and who is to do it. It bridges the gap between where we are and where we want to go.
- Organising: It includes division of work among employees by assigning each employee their duties, delegation of authority as required.
- Procurement: Acquisition deals with job analysis, human resource planning, recruitment, selection, placement, transfer and promotion.
- Development: Development includes performance appraisal, training, executive development, career planning and development, organisational development.
- Compensation: It deals with job evaluation, wage and salary administration, incentives, bonuses, fringe benefits and social security schemes.
IV. Long Answer Questions.
Question 1.
Explain the characteristics of Human Resource. [OWE] [MOM] [LACE] (any 5) Characteristics
Answer:
- HR is the Only factor of production that lives.
- HR can Work as a team.
- HR Exhibits innovation and creativity.
- HR is Movable.
- HR Over years – gain values.
- HR can Motivated either in cash or in kind.
- HR the Labour of employees that is hired and not the employee himself.
- HR alone Act, Analyse, Think and interpret.
- HR Create all other resources.
- HR are Emotional beings.
![]()
Question 2.
Describe the significance of Human Resource Management. (MEERA) (JCE) (any 5)
Answer:
The role of human resource management is the process of acquiring, training, appraising, and compensating employees. The significance of human resource management is given below:
- To identify manpower needs: Determination of manpower needs in an organisation is very important as it is a form of investment.
- To ensure the correct requirement of manpower: At any time the organisation should
not suffer from shortage or surplus manpower which is made possible through human resource management - To select the right man for the right job: Human resource management ensures the right talent to select the right employee for the right job.
- To update the skill and knowledge: Human resource management enables employees to remain up-to-date through training and development programmes.
- To appraise the performance of employees: A periodical appraisal of the performance of employees through human resource management activities boosts up good performers and motivates slow performers.
![]()
Question 3.
Elaborate on the Managerial functions of Human Resource Management.
Answer:
Managerial Functions : [COP] [D]
Controlling”
- It is comparing the actuals with the standards and to check whether activities are going on as per plan and rectify deviations.
- The control process includes fixing the standards, measuring actual performance, comparing the actual with standard laid down, measuring deviations and taking corrective decisions.
- This is made possible through observation, supervision, reports, records and audit.
Organising:
- It includes division of work among employees assigning duties to each employee.
- Delegation of authority as required, creation of accountability to make employees responsible.
Planning:
- Planning is thinking before doing.
- Planning is deciding in advance What to do, How to do, Who is to do it.
- It bridges the gap between where we are and where we want to go.
- It involves determination of objectives, policies, procedures, rules, strategies, programmes and budgets.
Directing:
- It involves issue of orders and instructions along with supervision, guidance and motivation to get the best out of employees.
- This reduces waste of time, energy and money and early attainment of organisational objectives.
Question 4.
Discuss the Operative functions HRM.
Answer:
Operating functions of HRM:
- Procurement: Acquisition deals with job analysis, human resource planning, recruitment, selection, placement and promotion.
- Development: It includes performance appraisal, training, executive development, and organizational development.
- Compensation: It deals with job evaluation, wage and salary administration, incentives, bonus schemes.
- Retention: This is made possible through health and safety, social security, job satisfaction and quality of work life.
- Maintenance: This encourages employees to work with job satisfaction, reducing labour turnover, for human resource.
![]()
12th Commerce Guide Human Resource Management Additional Important Questions and Answers
I. Choose the correct answer.
Question 1.
create all other resources.
a) Skill
b) workforce
c) Natural resources
d) Human resource
Answer:
d) Human resources
Question 2.
The function of HRM concerned with …………………………
a) Motivating
b) Maintaining
c) Hiring
d) All of these
Answer:
d) All of those
![]()
Question 3.
How to encourage the best performer in an organisation?
a) Bonus
b) incentives
c) Both (a) and (b)
d) NOTA
Answer:
c) Both (a) and (b)
Question 4.
Human Resource Exbhits ……………………
a) Talent
b) Creativity
c) Innovation
d) All of those
Answer:
d) All of those
Question 5.
Who are inevitable for the survival and success of the enterprise?
a) Employee
b) Proprietors
c) Debtors
d) Creditors
Answer:
a) Employee
![]()
Question 6.
HR is ………………
a) Im-morable
b) In-tangble
c) Movable
d) NOTA
Answer:
c) Movable
II. Match the following
Question 1.
Match List I with List – II
List -i | List – ii |
| (i) HR | 1.Right man Right Job |
| (ii) HRM | 2.Integration |
| (iii) Operative Function | 3. Planning |
| (iv) Managerial Function | 4. Employers |

Answer:
a) (i) 4,(ii) 1,(iii) 2,(iv) 3
![]()
III. Very short answer questions.
Question 1.
Define the term Human Resource.
Answer:
Man of all sources available to him, can grow and develop”.
Question 2.
Give two points of difference between HR and HRM .[MO]
Answer:
Basis of Difference | HR Human Resource | HRM |
| 1.Motivation | HR can be motivated in cash or kind. | HR goal is achieved and employers are satisfied. |
| 2. Organisational Goal | Formulate policies by giving individual needs and organisational needs. | HRM goal is achieved both employers and employees are satisfied. |
![]()
IV. Long Answer Questions.
Question 1.
Explain the features of HRM? [CUGAI]
Answer:
Continuous Process:
As long as there is HR in the running of an organisation, the activities relating to managing , HR exists.
Universal Relevent:
- HRM has universal relevance.
- The approach and style varies depending the nature of organisation structure and is applicable at all levels.
Goal Oriented :
The accomplishment of organisational goals is made possible through best utilisation of HR is an organisation.
A Systematic Approach:
HRM lavs emphasis on a systematic approaching in managing the tasks performed by HR of an organisation.
Intangible:
HRM is an intangible function which can be measured only by results.
![]()
Question 2.
Differentiate HR from HRM.[MOPET]
Answer:
Basis of Difference | HR Human Resource | HRM |
| 1. Motivation | HR can be motivated in cash or kind. | Formulate policies by giving individual needs and organisational needs. |
| 2. Organisational Goal | HR goal is achieved and employers are satisfied. | HRM goal is achieved both employers and employees are satisfied. |
| 3. Production | HR is the only factor of production. | HRM has universal relevance. |
| 4. Exhibits | HR exhibits innovation and creativity. | HRM is strategic function. |
| 5. Think | HR alone think, act, analyse and interpret. | HRM is development oriented. It provides space for employee involvement, performance and growth. |






 .
.


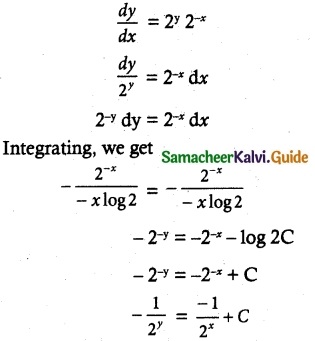
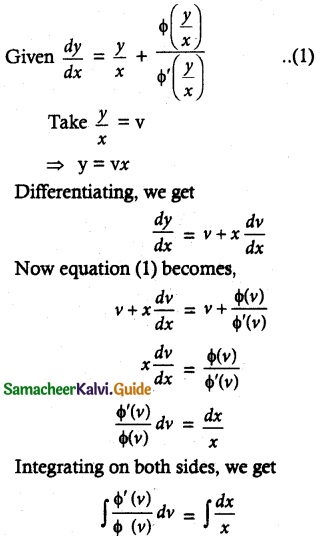
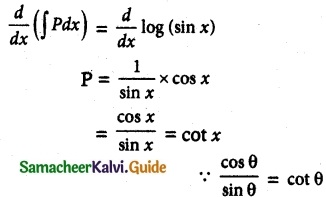
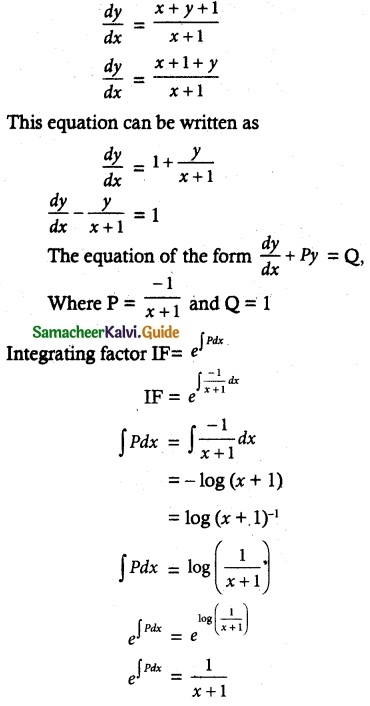
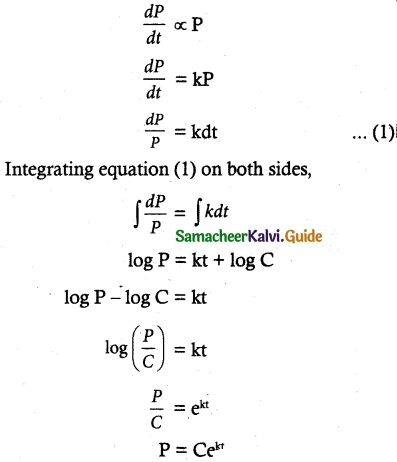
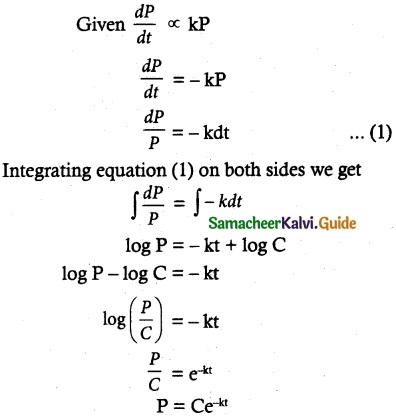
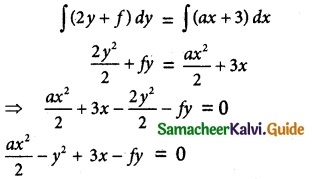
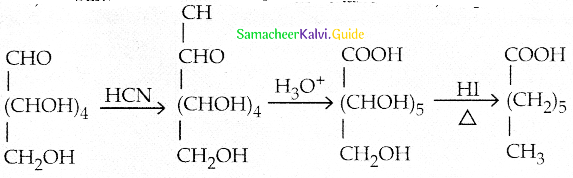
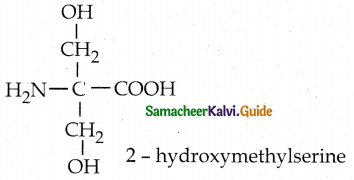
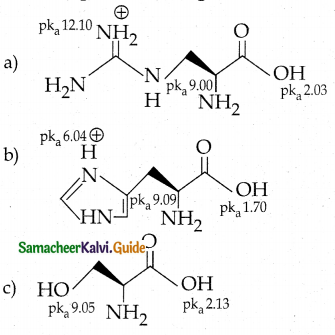
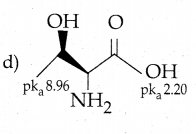
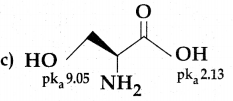
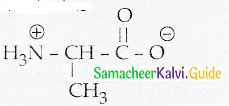

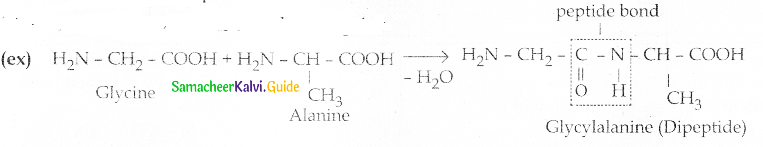
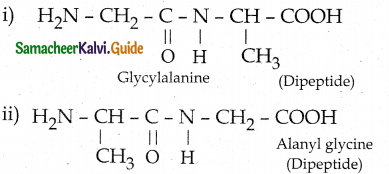
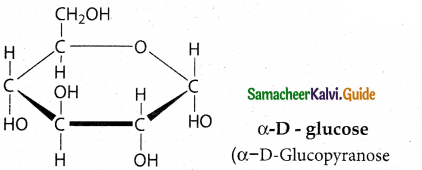
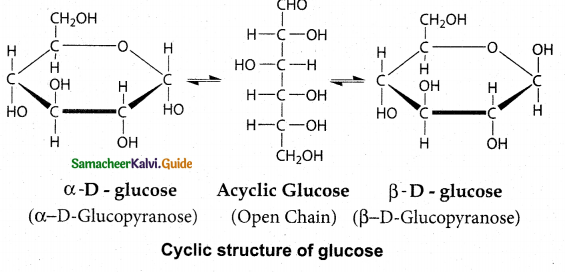
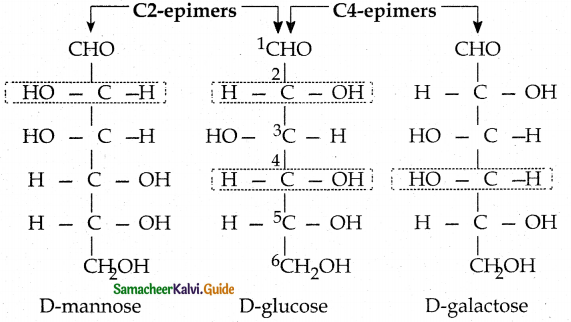
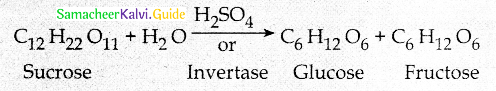
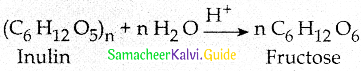
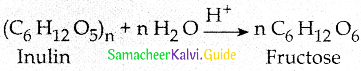
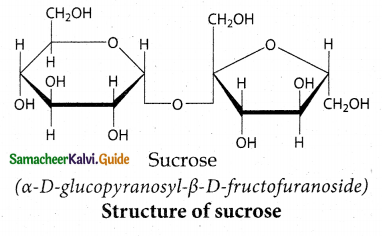
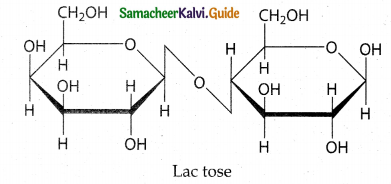
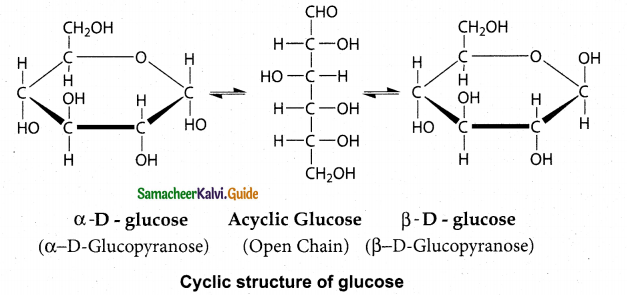
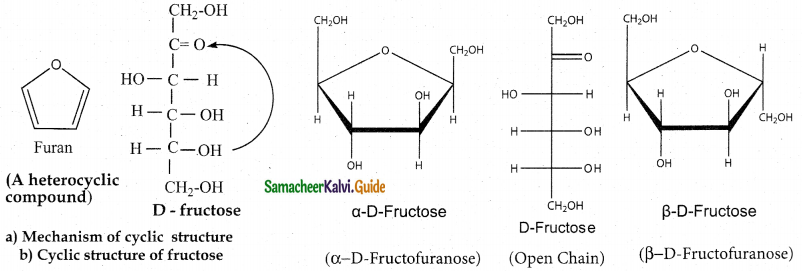
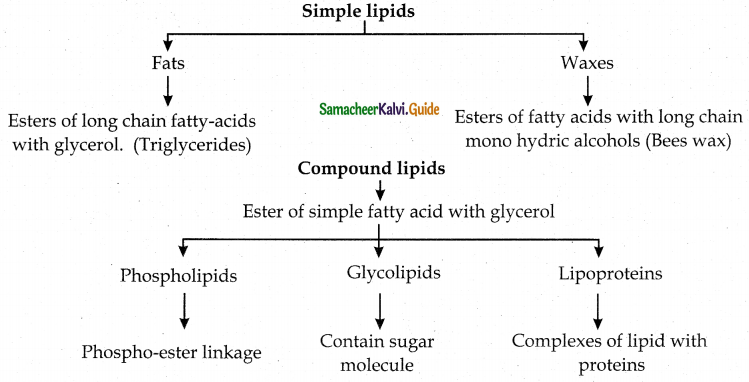
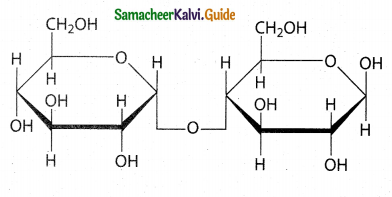 Maltose
Maltose