Tamilnadu State Board New Syllabus Samacheer Kalvi 12th Commerce Guide Pdf Chapter 10 Recruitment Methods Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 12th Commerce Solutions Chapter 10 Recruitment Methods
12th Commerce Guide Recruitment Methods Text Book Back Questions and Answers
![]()
I. Choose The Correct Answer.
Question 1.
Recruitment is the process of identifying ………………..
a) Right Man for Right Job
b) good performer
c) Right job
d) All of the above
Answer:
a) Right Man for Right Job
Question 2.
Recruitment bridges gap between …………. and ………..
a) job seeker and job provider
b) job seeker and agent
c) job provider and owner
d) owner and servant
Answer:
a) job seeker and job provider
Question 3.
Advertisement is a …………………… source of recruitment.
a) internal
b) external
c) agent
d) outsourcing
Answer:
b) external
![]()
Question 4.
Transfer is an …………… source of recruitment.
a) internal
b) external
c) outsourcing
d) None of of the above
Answer:
a) internal
Question 5.
E – Recruitment is possible only through ……………….. facility.
a) Computer
b) Internet
c) Broadband
d) 4G
Answer:
b) Internet
![]()
II. Very short answer questions.
Question 1.
Give the meaning of Recruitment.
Answer:
Recruitment is the process of finding suitable candidates for the various posts in an organisation.
Question 2.
What is promotion?
Answer:
Based on the Seniority and merits of the employees they are given the opportunity to move up in the organisational hierarchy.
Question 3.
Write any two internal sources of recruitment.
Answer:
Benefits of an internal source of recruitment:
- The internal source will reduce the cost and expenses of recruitment.
- It is very useful, by way of selecting from the existing and retiring employees.
![]()
Question 4.
What is meant by campus recruitment?
Answer:
The organisations visit the educational institutions to identify and recruit suitable candidates.
Question 5.
What is meant by poaching?
Answer:
Organizations instead of training and developing their own employees hire employees of other competitive companies by paying them more both financial and non-financial benefits. It is also called raiding.
![]()
III. Short Answer Questions.
Question 1.
Define the term Recruitment.
Answer:
According to Edwin B. Flippo, “It is a process of searching for prospective employees and stimulating and encouraging them to apply for jobs in an organisation.”
Question 2.
What is meant by unsolicited applicants?
Answer:
These are the applications of job seekers who voluntarily apply for the vacancies not yet notified by the organisations.
![]()
Question 3.
What is meant by job portals?
Answer:
- A job portal is a web that links the gap between the employer and job seekers.
- Because of IT development, the employer can select the abled from throughout the world.
- Using Internet job portals organisation can screen for prospective candidates and fill up their vacancies.
Question 4.
State the steps in the Recruitment process, outsourcing.
Answer:
- Planning recruitment
- Determining vacancies
- Identifying the sources
- Drafting information for advertisement
- Selecting the suitable mode of advertisement
- Facilitating selection process
- Evaluation and control
![]()
IV. Long Answer Questions.
Question 1.
Explain the internal sources of recruitment (any 5).
Answer:
Internal Sources:
The following are the Internal Sources of recruitment.
(i) Transfer:
The simplest way by which employee recruitment can be filled is through the transfer of employees from one department with surplus staff to that of another with deficit staff.
(ii) Upgrading:
Performance appraisal helps in the process of moving employees from a lower position to a higher position.
(iii) Promotion:
Based on the seniority and merits of the employees they are given the opportunity to move up in the organisational hierarchy.
(iv) Demotion:
The movement of an employee from a higher position to a lower position because of poor performance continues to make him realise the significance of the performance.
(v) Job Rotation:
- One single employee managing to learn how to perform in more than one job on rotation.
- This familiarises the employees with all kinds of jobs performed and becomes a source.
![]()
Question 2.
Explain the External sources of recruitment. (any 5)
Answer:
External Sources:
a. Direct – Sources: (WAR)
Walk-ins: Walk-in applicants with suitable qualifications and requirements can be another source of recruitment.
Advertisement:
- The employer can advertise in periodicals – about the vacancies in the organisation.
- Specifying nature of work and vacancy.
- Qualification and experience required.
- Salary offered.
- Mode of applying – The last date to apply.
Rival firms:
- Where the efficient employers of rival companies are drawn to the organisation by higher pay and benefits.
- It is known as “Poaching” or “Attracting” or “Raiding” or “Hunting”.
- And other Direct – Sources are campus and e-recruitment, unsolicited applicants, Factory Gate recruitment, etc.
b. Indirect – Sources : [JEEP]
Job portals: Using internet job portals organisations can screen for prospective candidates and fillup their vacancies.
Employee referral:
The existing employees of the organisation may recommend some of their relatives or known people who will be suitable for the existing vacancies.
Employment Agencies :
It is like a Public Employment Exchange, owned by the private parties and connect the job providers and the job seeker.
Public Employment Exchange :
It is established by the Government and bridging the gap between the Employer and the job seeker and makes available the information through a database. And other indirect sources are Labour Contractors Poaching Outsourcing, Deputation, etc. Word of mouth.
![]()
Question 3.
What are the Recent Trends in Recruitment?
Answer:
The recent methods of recruiting by organisations include the following methods:
1 . Outsourcing – There are outsourcing firms that help in the process of recruiting through screening of applications and finding the right person for the job for which job they are paid service charges.
Recruitment Process Outsourcing:
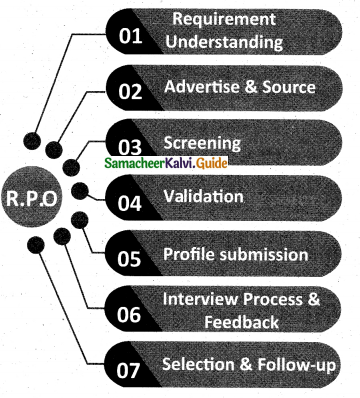
2. Poaching – Organisations instead of training and developing their own employees hire employees of other competitive companies by paying them more both financial and non-financial benefits. It is also called raiding.
12th Commerce Guide Recruitment Methods Additional Important Questions and Answers
I. Choose The Correct Answer.
Question 1.
Promotion is a …………………….. source of requirement.
a) internal
b) external
c) modern
d) NOTA
Answer:
a) internal
Question 1.
The internal source of recruitment are _________
(i) promotion
(ii) e-recruitment
(iii) retention
(iv) advertisements
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (iii) and (iv)
Answer:
(b) (i) and (iii)
![]()
Question 3.
Poaching is also known as ………………
a) Selection
b) Yield
c) Success
d) Raiding
Answer:
d) Raiding
Question 4.
The process of attracting potential people to apply for a job in an organisation is ………………
a) Selection
b) Recruitment
c) HR
d) NOTA
Answer:
c) E-recruitment
![]()
Question 5.
…………………………….. helps in the process of moving employees from a lower position to a higher position.
a) Appraisal
b) Knowledge
c) Skill
d) All of these
Answer:
d) All of these
Question 6.
Movement of an employee from a higher position to a lower position because of poor performance is known as ………………
a) Promotion
b) Demotion
c) Rotation
d) Retention
Answer:
b) Demotion
![]()
Question 7.
The organisation visits the Educational institutions to recruit suitable candidates is known as……………
a) Job Portals
b) Campus
c) Word of Mouth
d) Online
Answer:
b) Campus
Question 8.
Why vacancy arises?
a) Death
b) Retirement
c) Resignation
d) All of these
Answer:
d) All of these
![]()
Question 9.
Applicants with suitable qualification requirement can be known as …………..
a) Walk-in
b) Campus
c) Advertisement
d) NOTA
Answer:
a) Walk-in
Question 10.
Pick the odd one out:
a) Rival firms
b) Walk-ins
c) Campus
d) Deputation
Answer:
d) Deputation
![]()
Question 11.
Pick the odd one out:
a) Word of mouth
b) Job portals
c) Labour contractors
d) Campus
Answer:
d) Campus
Question 12.
Pick the odd one out:
a) Transfer
b) Promotion
c) Demotion
d) Advertisement
Answer:
d) Advertisement
![]()
Question 13.
Pick the odd one out:
a) Planning
b) Determining
c) Identifying
d) Upgrading
Answer:
d) Upgrading
Question 14.
Which one of the following is correctly matched?
a) E-Recruitment – Online methods
b) Unsolicited Applicants – Applications of job seekers
c) Employee referral – Existing employees recommendation
d) Private Employment Agencies – Established by the Government
Answer:
d) Private Employment Agencies – Established by the Government
![]()
Question 15.
Which is the correct statement?
The vacancy arises due to:
(i) Retirement or Death of an employee.
(ii) Resignation of an employee.
(iii) Disablement and Dismissal of an employee.
a) (i) is correct
b) (ii) is correct
c) (iii) is correct
d) All (i), (ii) and (iii) are correct
Answer:
d) All (i), (ii) and (iii) are correct
![]()
II. Match The Following
Question 1.
Match List I with List – II
| List-I | List-II |
| (i) Promotion | 1. Retiring employee be used |
| (ii) Demotion | 2. How to perform in more than one job |
| (iii) Retention | 3. Higher position to lower position |
| (iv) Job Rotation | 4. Lower position to higher position |

Answer:
a) (i) 4, (ii) 3, (iii) 1, (iv) 2
Question 2.
List-I | List-II |
| (i) Transfer | 1. Already applied |
| (ii) Dependants | 2. Acquires another business |
| (iii) Previous Applicants | 3. One department to another |
| (iv) Acquisition and Mergers | 4. Legal hairs of the deceased employee |

Answer:
b) (i) 3, (ii) 4, (iii) 1, (iv) 2
Question 3.
List -I | List -II |
| i. Poaching | 1. Temporary and unskilled employees |
| ii. Factory Gate | 2. Efficient employees of rival firms |
| iii. Campus | 3. Applicants with suitable Qualification |
| iv. Walk-ins | 4. Visits the educational institutions |

Answer:
a) (i) 2,(ii) 1,(iii) 4,(iv) 3
III Assertion And Reason.
Question 1.
Assertion (A): One single employee managing to learn how to perform in more than one job on rotation.
Reason (R): It familiarises the employees with all kinds of jobs performed and becomes a source. ,
a) (A) is True (R) is False
b) (A) is False (R) is True
c) Both (A) and (R) are True
d) Both (A) and (R) are False
Answer:
c) Both (A) and (R) are True
IV. Very Short Answer Questions.
Question 1.
What is Retention?
Answer:
The retiring employees can be used to meet the requirement after extension as per management discretion.
Question 2.
How the Dependants are appointed?
Answer:
The legal heirs or The Dependant employees may be given a chance to replace the deceased.
Question 3.
What is meant by retention?
Answer:
Retention is a method of an internal source of recruitment. The retiring employees can be used to meet the requirement after superannuation as per management discretion.
Question 4.
What is Campus Recruitment?
Answer:
The organisations visit the Educational Institutions to identify and recruit suitable candidates.
Question 5.
What is E-Recruitment?
Answer:
The organisations which carryout recruitment online method is said to follow E-recruitment.
Question 6.
What is Deputation?
Answer:
A person who is already an employee of an organisation can be deputed for a specific job for a specified period to another organisation as a short-term solution.
Question 7.
What is word of mouth?
Answer:
The information relating to job seekers is collected through repute who pass on the message about the vacancy to their known people.
Question 8.
What is outsourcing?
Answer:
There are Outsourcing firms that help in the process of recruiting through screening of applications and finding the right Peron for the job for Which job they are paid service charge.
Question 9.
Mention any two Features of campus recruitment.
Answer:
- Most of the applicants are assembled in one place.
- The company can arrange it in a short period.
Question 10.
List the benefits of external sources of recruitment.
- The company receives new abilities and skills.
- New employees can change the habit of old employees.
V. Short Answer Questions.
Question 1.
What do you mean by poaching?
Answer:
Poaching means that the company can hire employees from other companies by paying them more. So the company can reduce the expense of giving training and developing their own employees. It is also called raiding.
Question 2.
| No. Basis of Difference | Advertisement | Unsolicited application |
| 1.Periodicals | In the advertisement, the employer can advertise in periodicals. [Dailies, Weeklies, Monthlies, etc.] | The job seekers voluntarily apply for the vacancies |
| 2.Vacancies | Vacancies are notified in the advertisement. | Vacancies are not notified. |
VI. Long Answer Questions.
Question 1.
What are the Internal Sources of Recruitment? [PUT] [DR]
Answer:
- Promotion: Based on seniority and merits of employees moving from lower position to higher position. [organisational hierarchy].
- Upgrading: Performance appraisal helps in the process of moving employees from lower positions to higher positions.
- Transfer: Transfer of an employee from one department with surplus staff to that of another with deficit staff.
- Demotion: Movement of employees from a higher position to a lower position because of poor performance.
- Retired employees: The employees who have already retired can be called to fill the vacancy as they have the required qualification and experience.
Question 2.
Explain the different methods of recruitment.
Answer:
There are basically two ways (methods) by which an organisation can recruit its employees.
- Internal Source.
- External Source.
External Sources can be further classified into:
- Direct Sources
- Indirect Sources
Internal Sources:
- Promotion – Job rotation
- Upgrading – Acquisition and Mergers
- Transfers – Retired employees
- Demotion – Dependents
- Recommodation by existing employees.
External Sources:
(a) Direct Sources: [WAR] [CUF] [E]
- Walk-ins – Campus Recruitment
- Advertisement – Unsolicited Applicants
- Rival firms – Factory gate Recruitments.
- E-recruitment
(b) Indirect Sources: [JEEP] [LOW] [PGD}
- Job portals
- Employee referral
- Employment – Consultancies – Agencies
- Professional Association
- Labour contractors
- Outsourcing
- Word of mouth
- Poaching [Hunting – Attracting Raiding]
- Government (public) Employment exchange Deputation.
Question 3.
Elaborate on the factors affecting recruitment.
Answer:
Internal Factors: SIR IF
Internal Factors are the factors within the organisation that affect recruiting the personnel in the organisation.
They are:
- Size of the organisation
- Image of the organisation
- Recruitment policy
- Image of job
- Future plans
External Factors: LULLS
External Factors are the factors outside the organisation influence recruiting the personnel in the organisation.
They are:
- Labour Market.
- Unemployment situation.
- Labour Laws.
- Legal Consideration.
- Socio-economic factors.
Question 4.
Discuss the importance of Recruitment.
Answer:
- Recruitment is the process of finding suitable candidates for various posts.
- It is the process of attracting people to apply for a job.
- It is bridging the gap between job provider and job seeker.
- It encourages prospective employees to apply for a job.
- It involves the communication of vacancies.
- It is an economical method.
- Its process is very simple.
- It is a positive one.
- No contractual relationship is established.
- It requires less time.

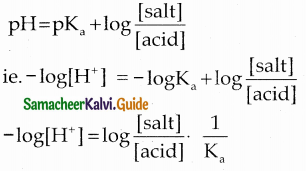
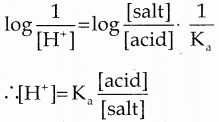
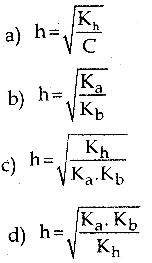
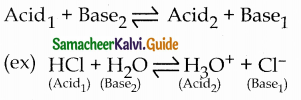
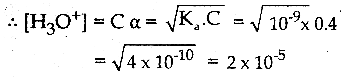
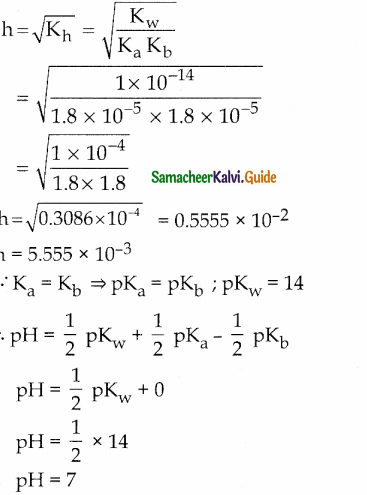
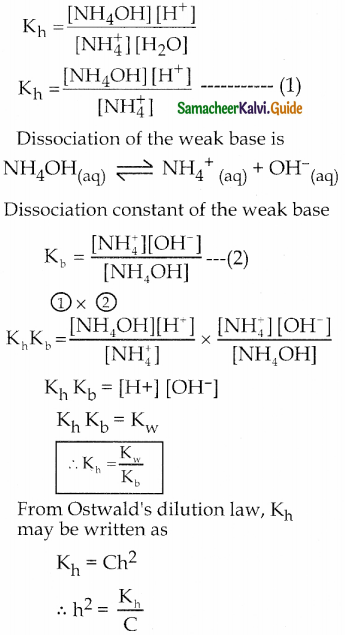
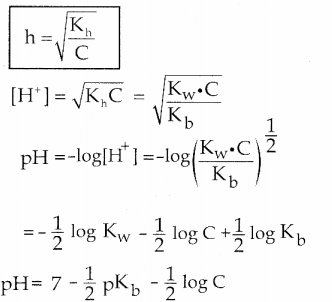
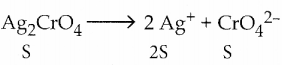
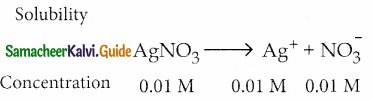
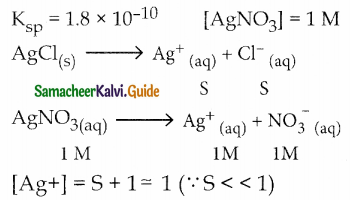
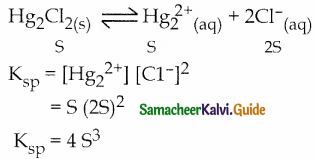
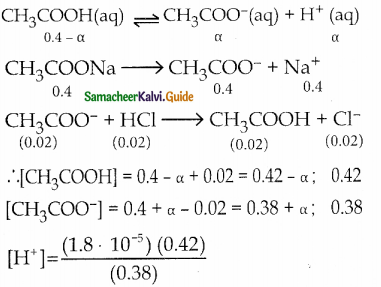

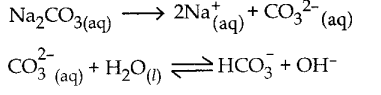
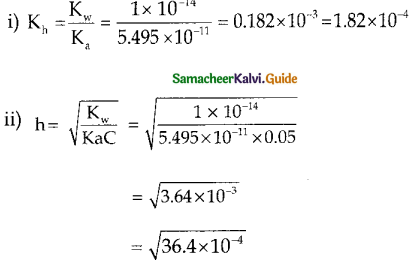
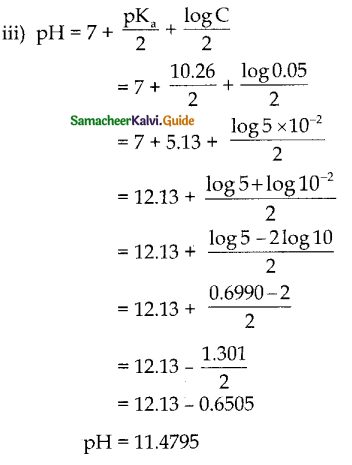
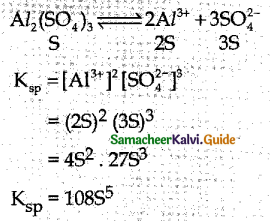
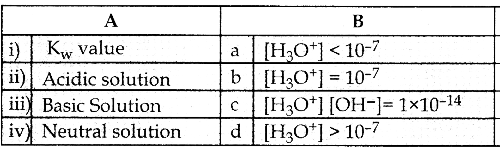
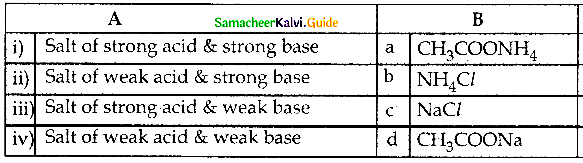
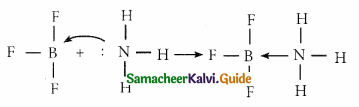
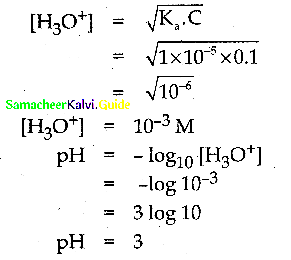
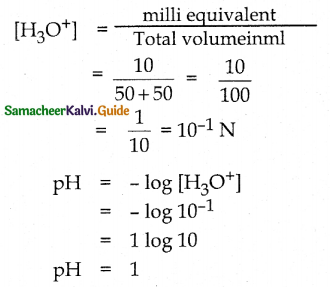
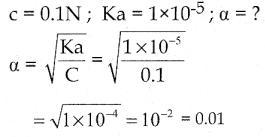
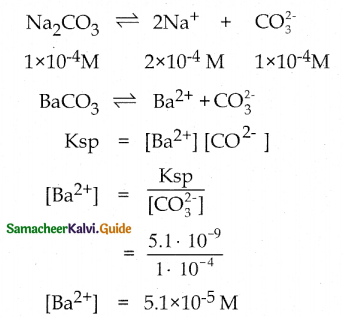
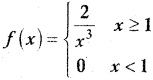
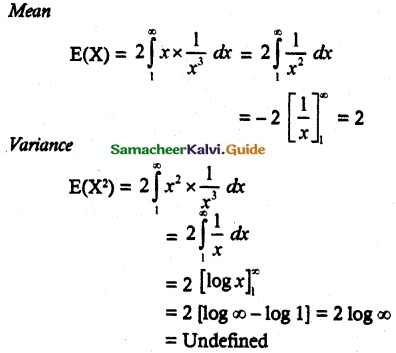
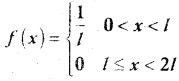
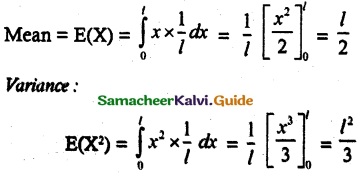
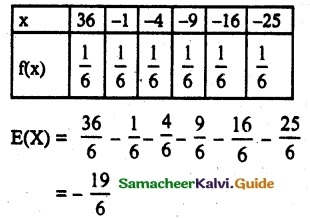
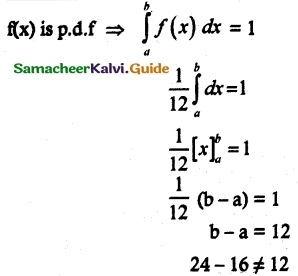
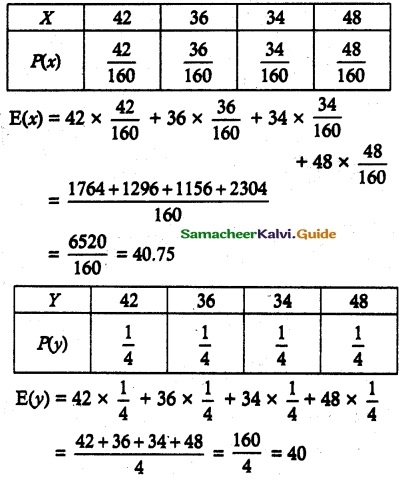

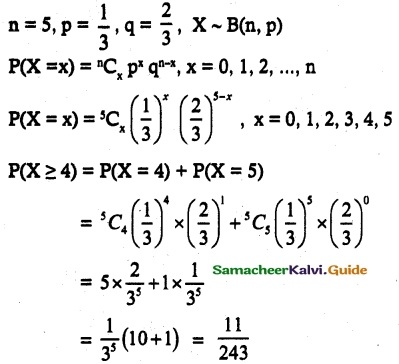
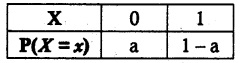
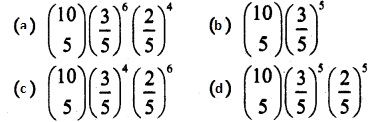
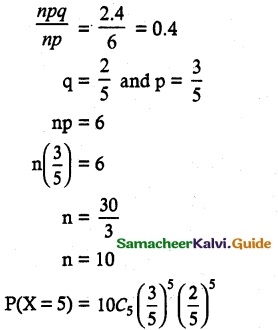
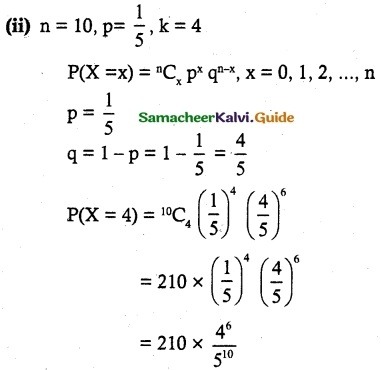
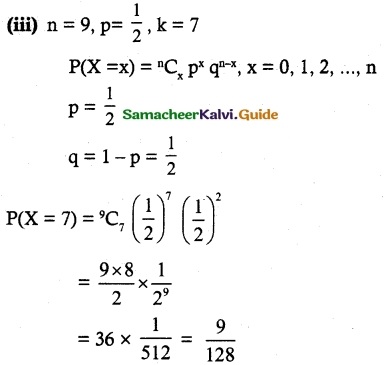
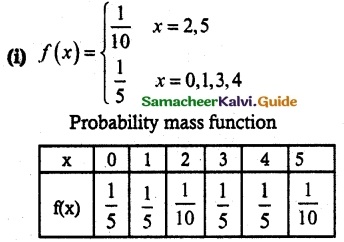
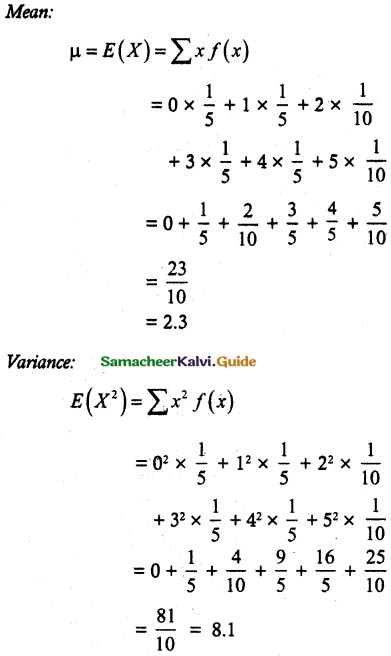
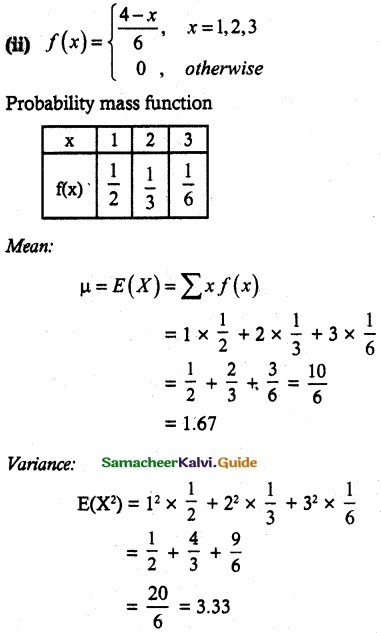
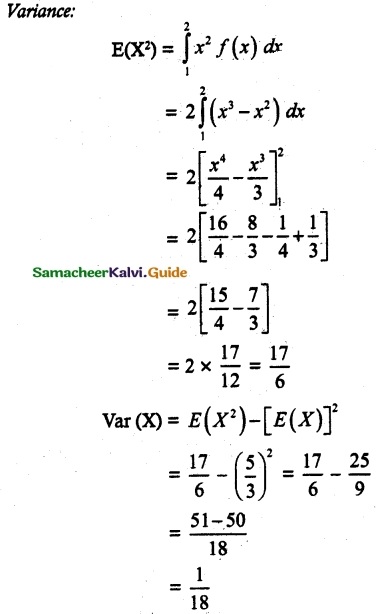
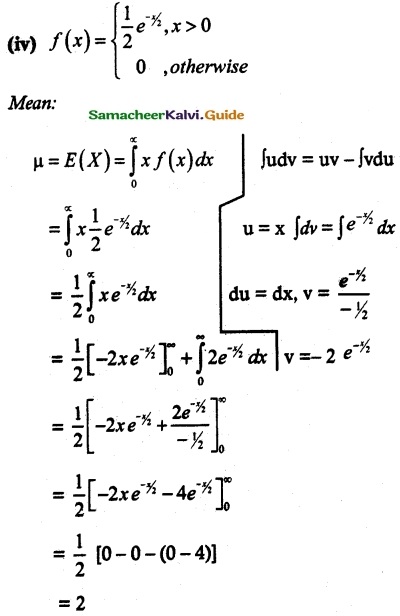
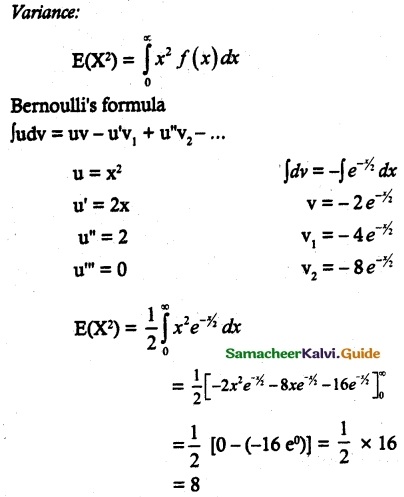
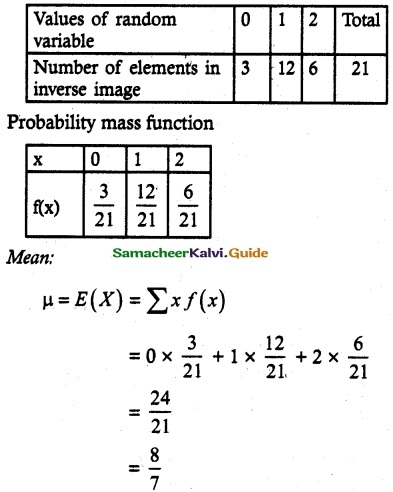
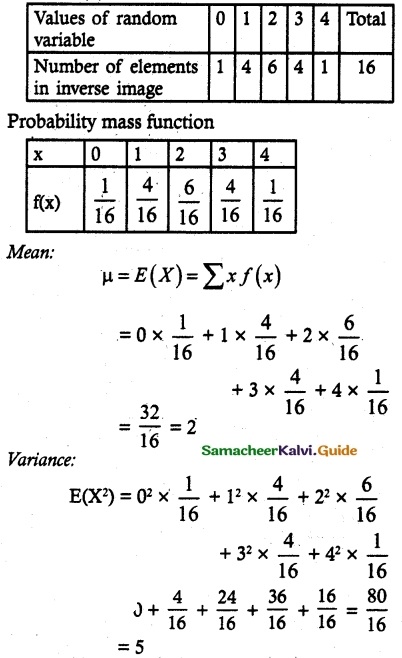
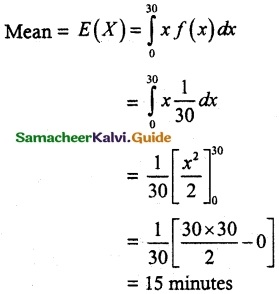
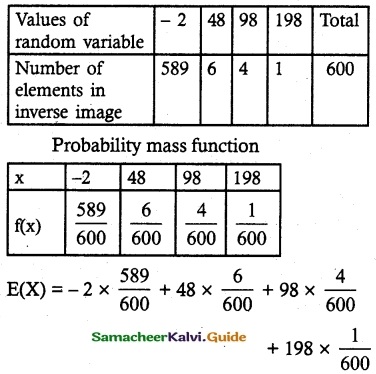
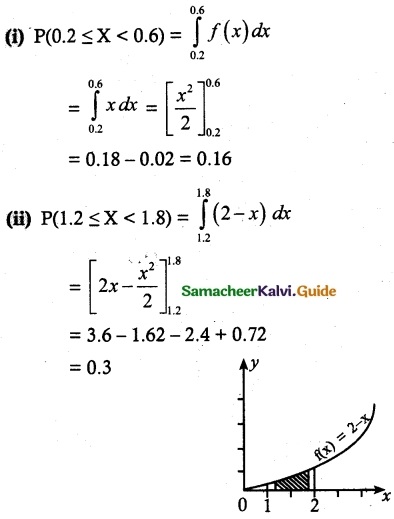
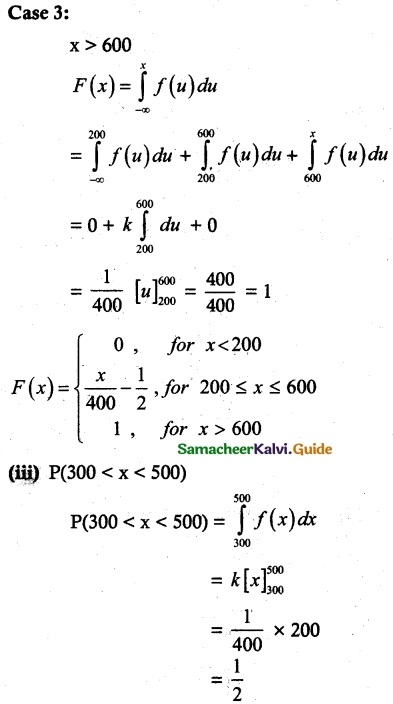
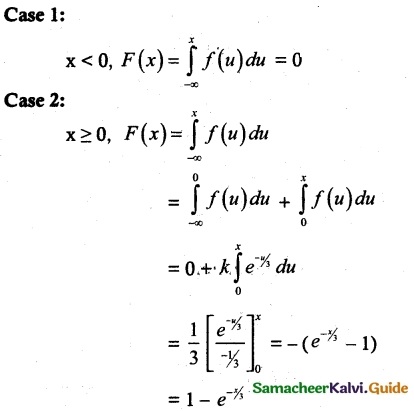
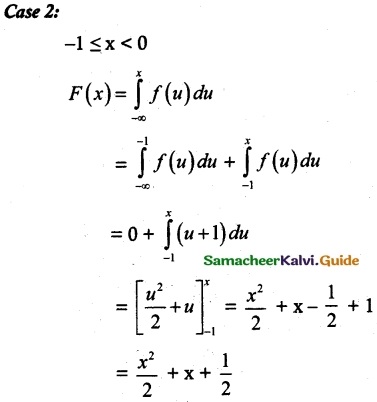
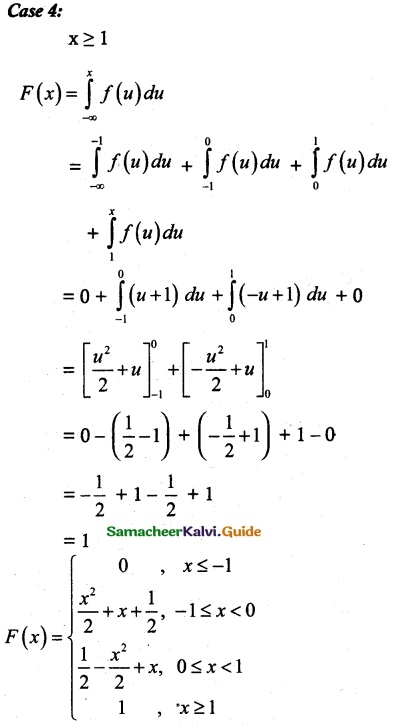
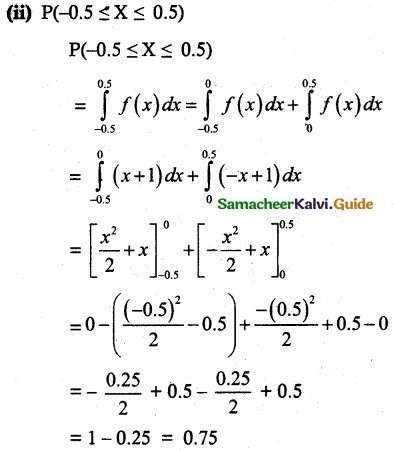
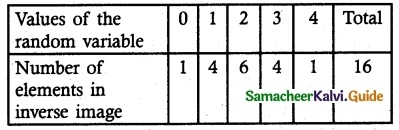
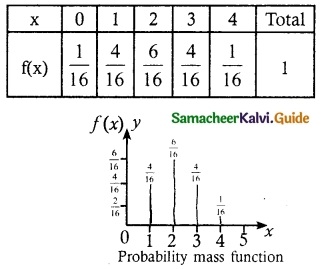
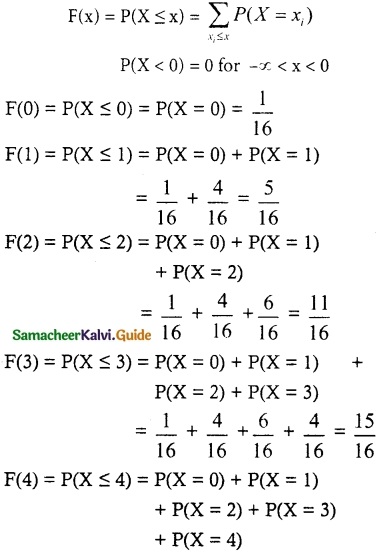
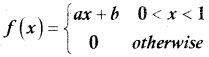 and E(X) = \(\frac { 7 }{ 12 }\) then a and b are respectively
and E(X) = \(\frac { 7 }{ 12 }\) then a and b are respectively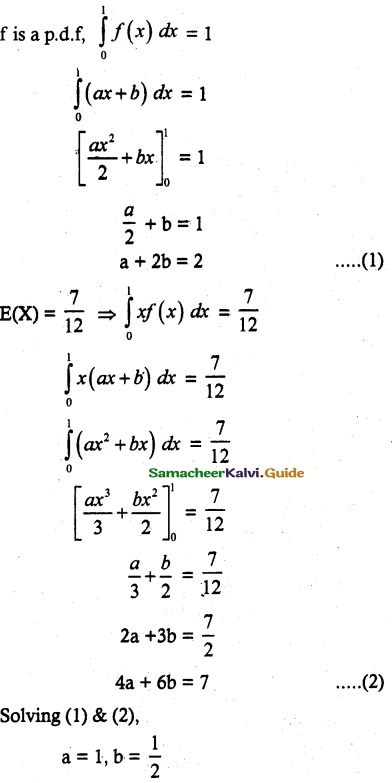
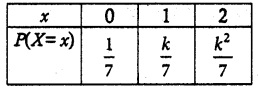
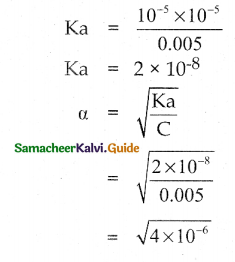
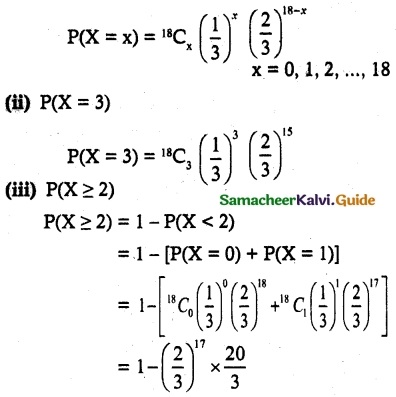
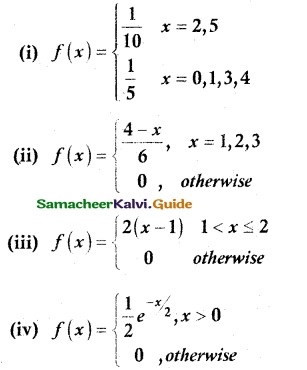
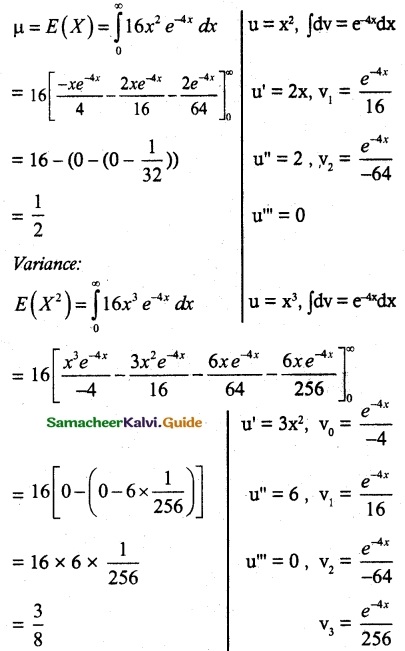
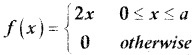 is a probability density function of a random variable, then the value of a is
is a probability density function of a random variable, then the value of a is
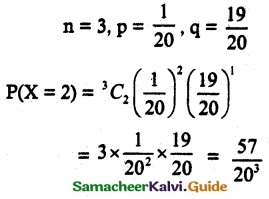
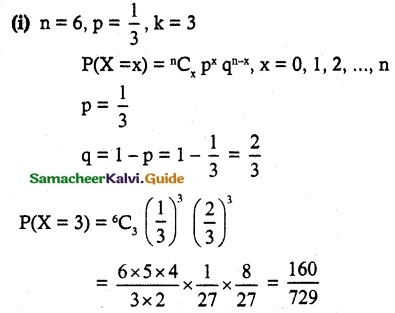
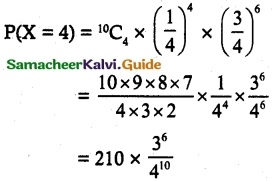
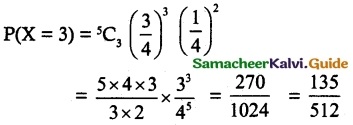
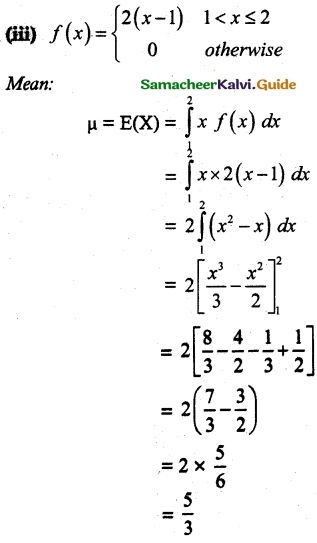
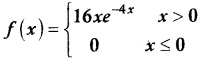
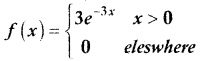
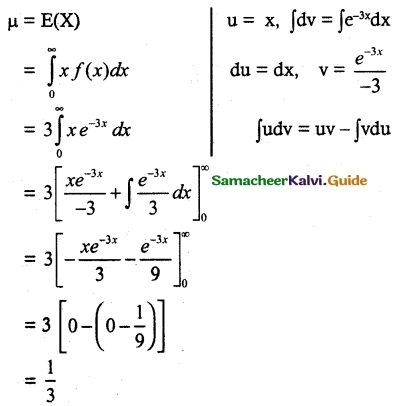
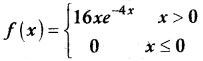

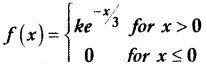
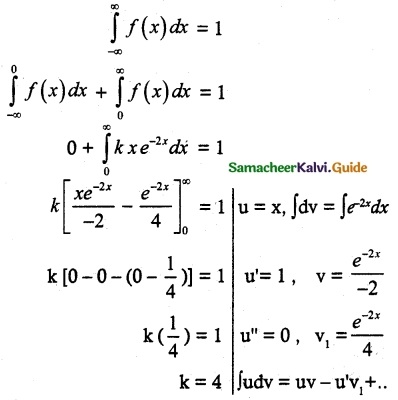
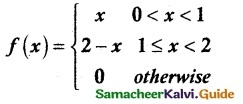
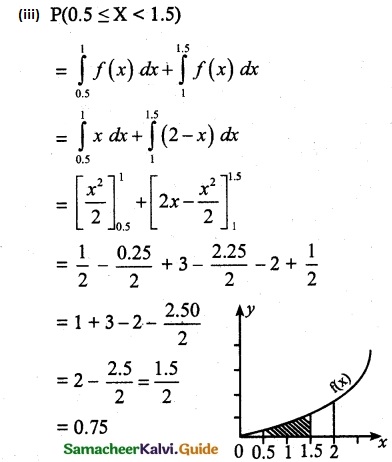
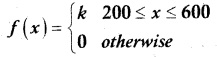
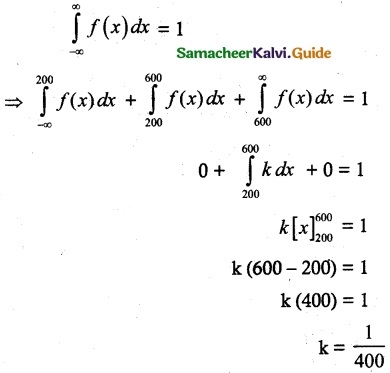
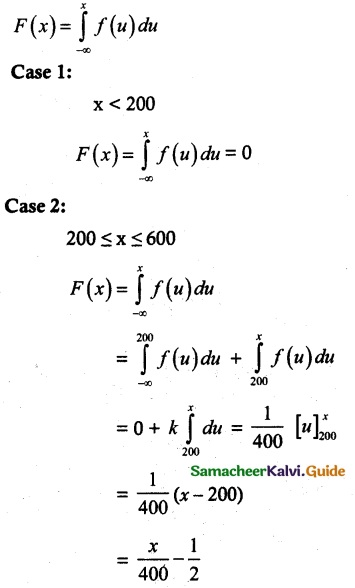
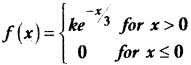
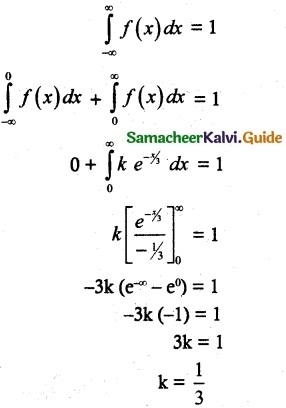
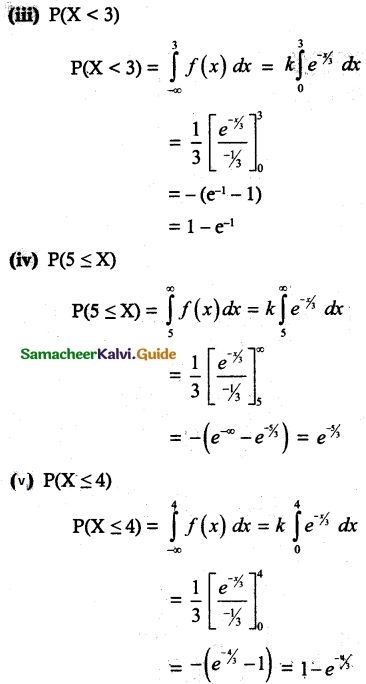
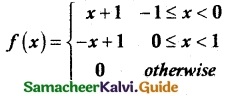
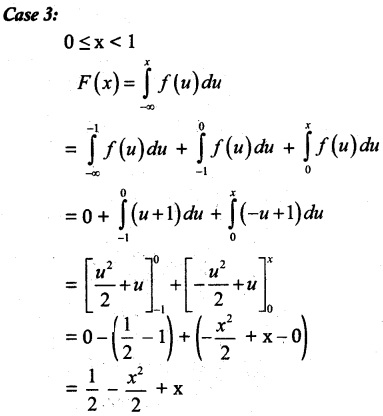

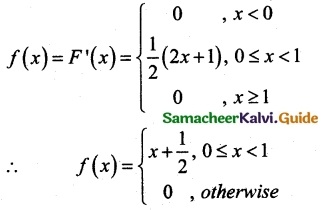
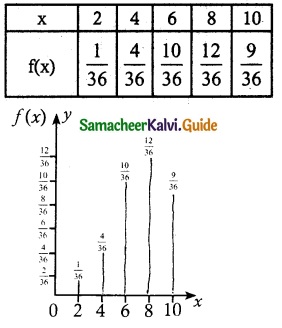
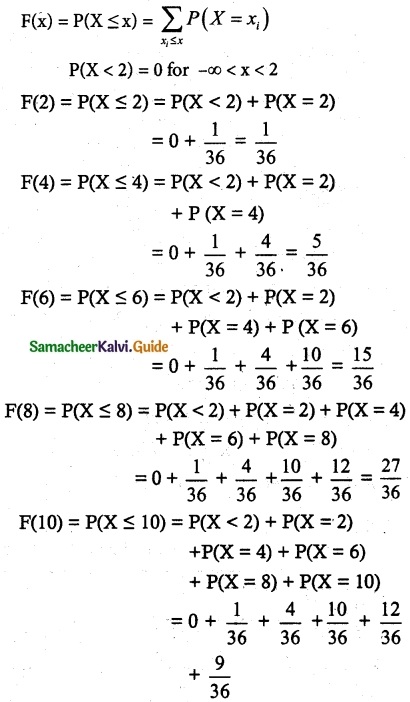
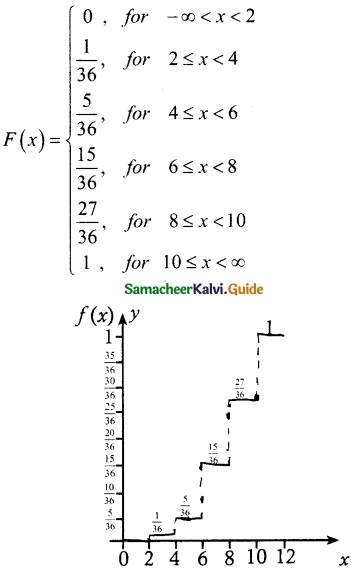
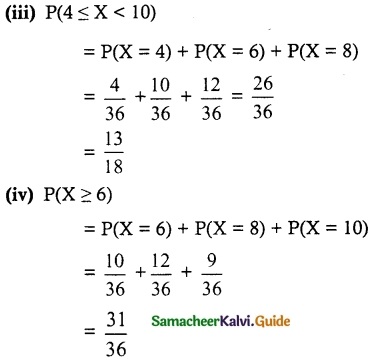
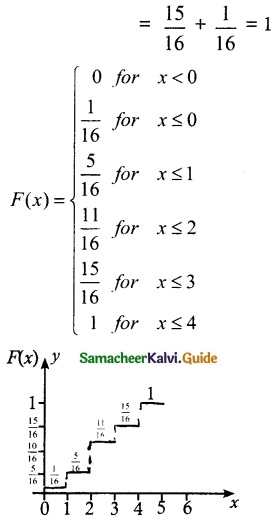
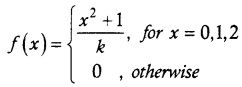
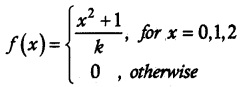
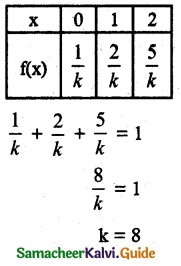
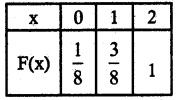
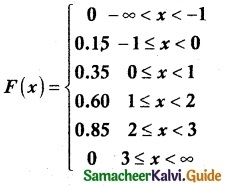
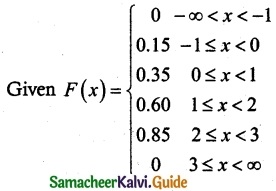
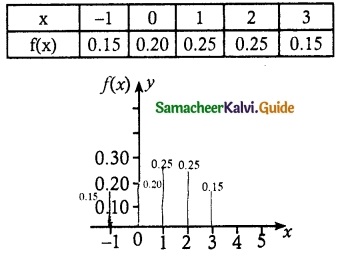

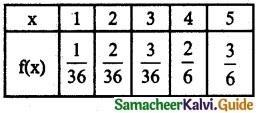
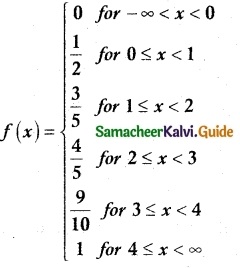
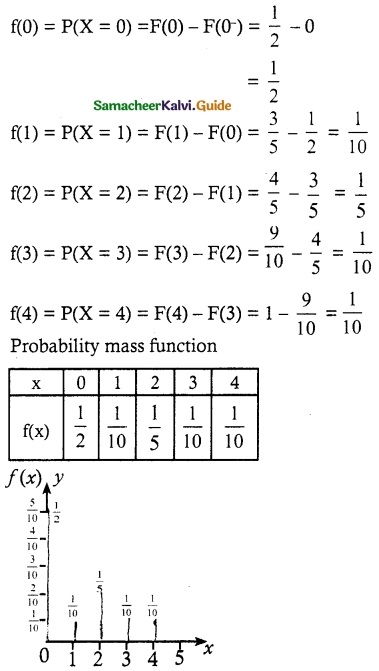




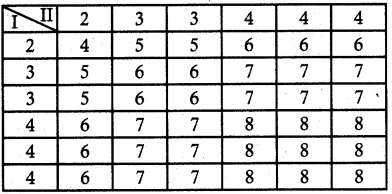
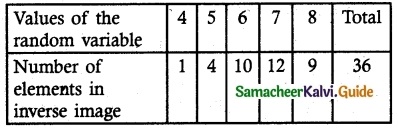
 .
.