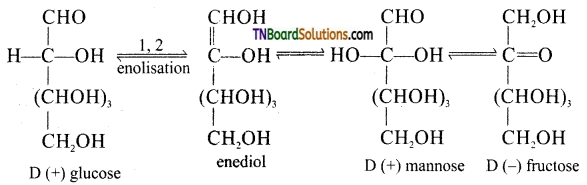
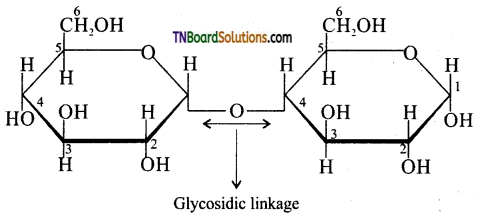
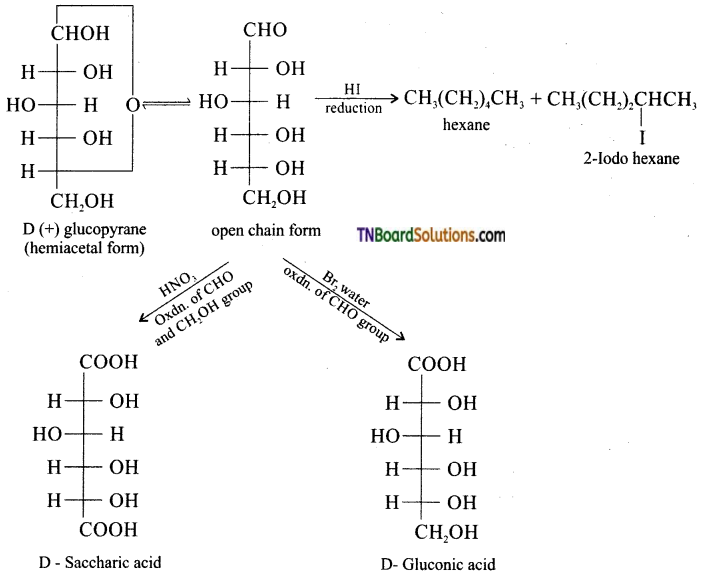
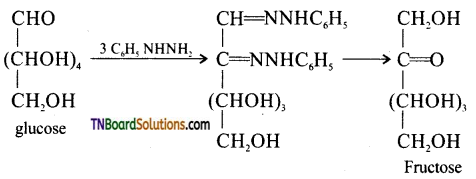
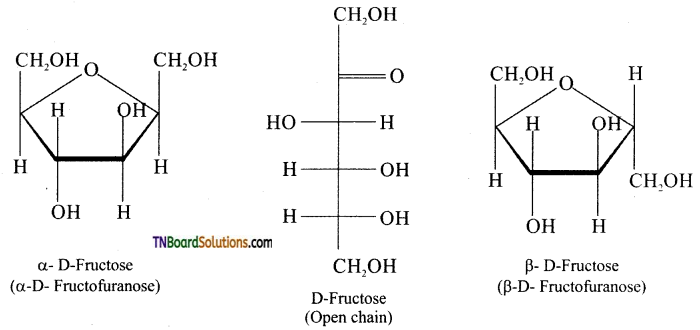
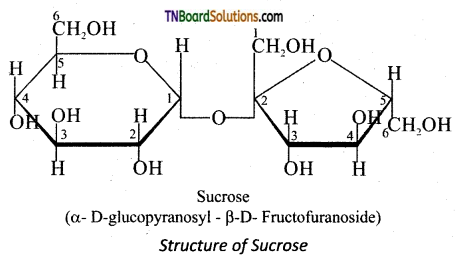
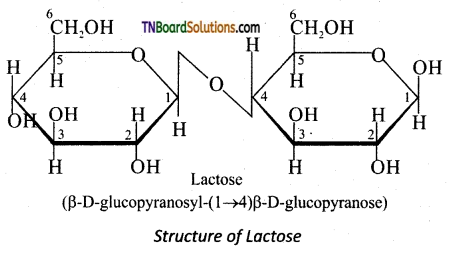
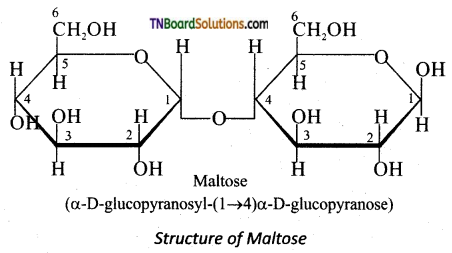
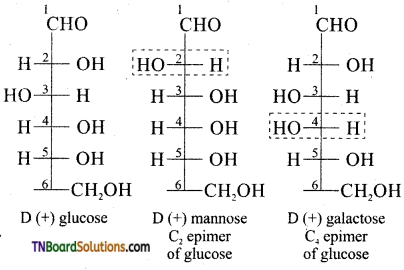
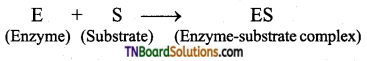
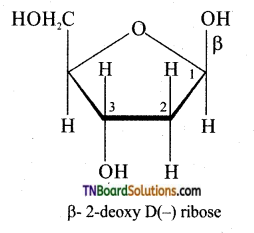
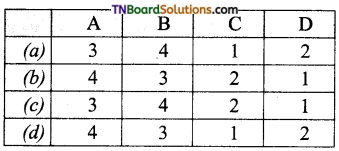
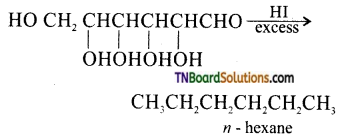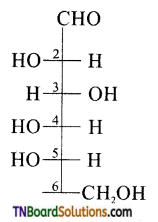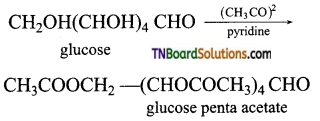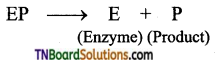Subject Matter Experts at TNBoardSolutions.Com have created Tamil Nadu State Board Samacheer Kalvi 3rd English Book Answers Solutions Guide Pdf Free Download of Term 1, 2, 3 are part of 3rd Standard Samacheer Book Solutions.
Let us look at these TN State Board New Syllabus Samacheer Kalvi 3rd Std English Guide Pdf of Text Book Back Questions and Answers Term 1, 2, 3, Chapter Wise Important Questions, Study Material, Question Bank, Notes and revise our understanding of the subject.
Tamilnadu State Board Samacheer Kalvi 3rd English Book Back Answers Solutions Guide Term 1, 2, 3.
Samacheer Kalvi 3rd Standard English Book Solutions Back Answers Guide
3rd Standard English Guide Samacheer Kalvi Term 1
Samacheer Kalvi 3rd Standard English Book Solutions Term 2
Samacheer Kalvi 3rd Standard English Book Answers Term 3
We hope these Tamilnadu State Board Samacheer Kalvi Class 3rd English Book Solutions Answers Pdf Free Download will help you get through your subjective questions in the exam.
Let us know if you have any concerns regarding TN State Board New Syllabus Samacheer Kalvi 3rd Standard English Guide Pdf of Text Book Back Questions and Answers Term 1, 2, 3, Chapter Wise Important Questions, Study Material, Question Bank, Notes drop a comment below and we will get back to you as soon as possible.
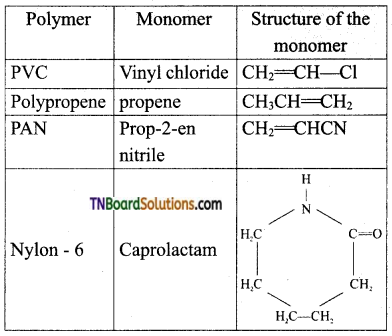
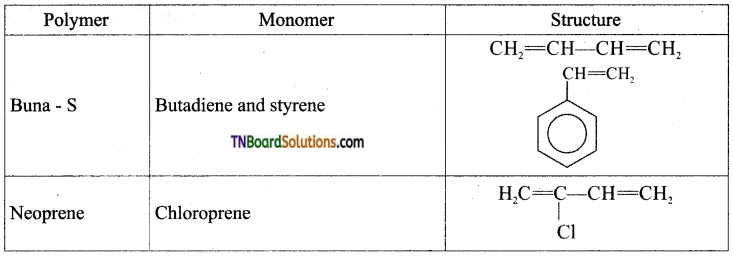
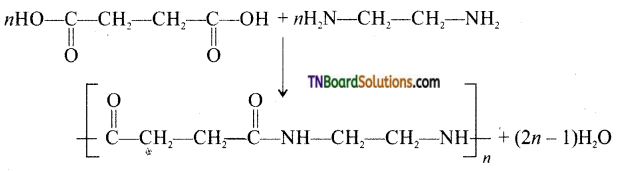
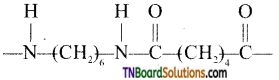
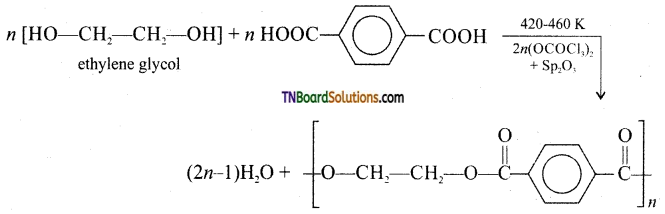
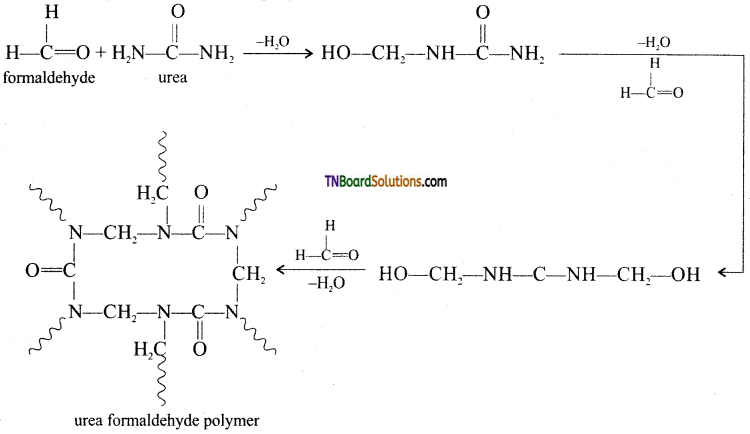
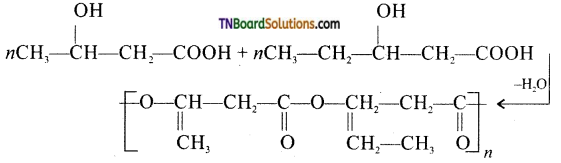
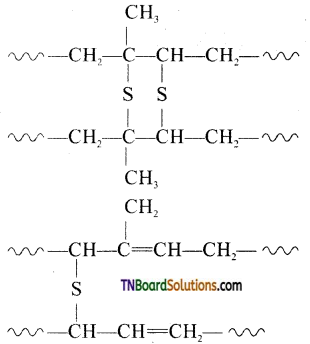
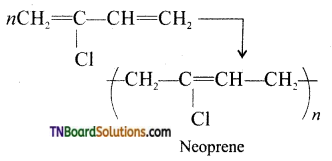
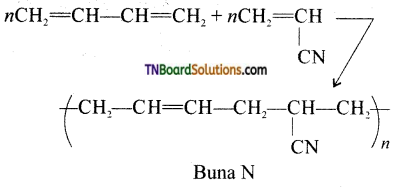
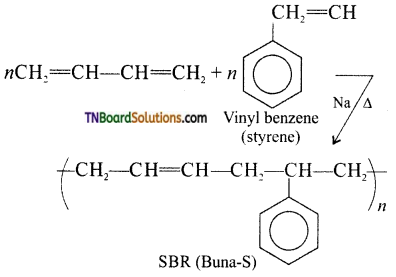
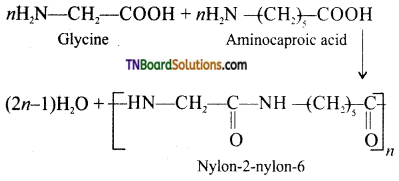
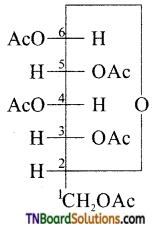
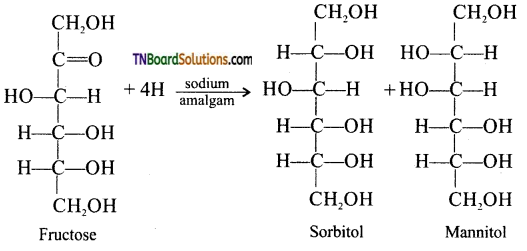
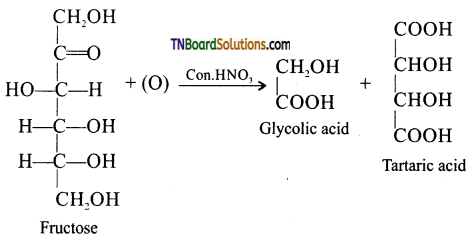
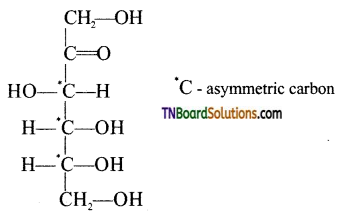
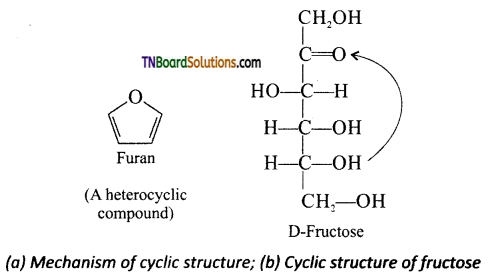
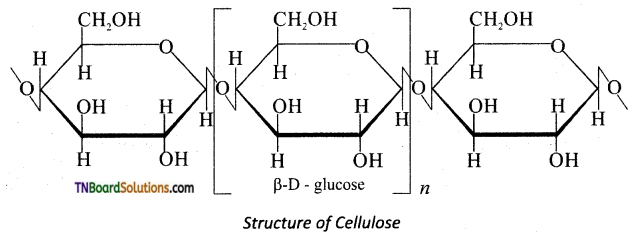
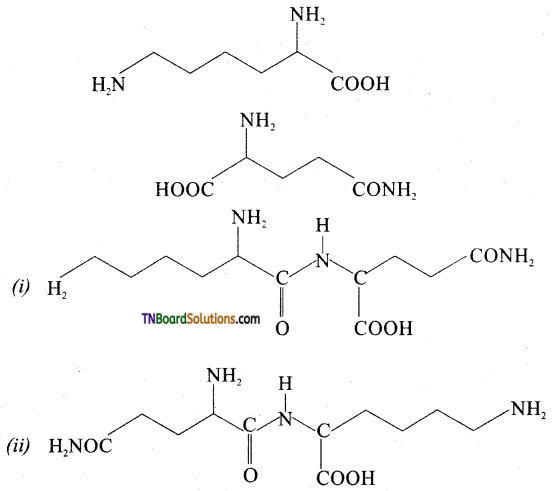
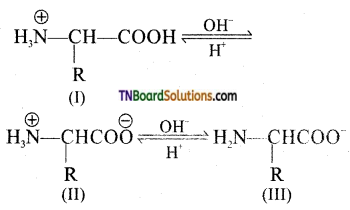
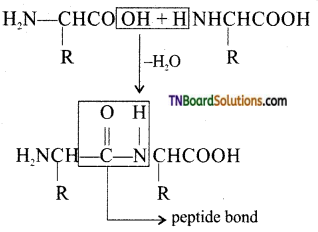
 which is derived from caprolactam.
which is derived from caprolactam.