Students get through the TN Board 12th Chemistry Important Questions Chapter 5 Coordination Chemistry which is useful for their exam preparation.
TN State Board 12th Chemistry Important Questions Chapter 5 Coordination Chemistry
Answer the following questions.
Question 1.
What is the coordination number of the central metal ions in the following complexes?
(i) [Cu(NH3)4]+2
(ii) [Fe(C2O4)3]-3
(iii) [Pt (en)2Cl2]
(iv) [Mo(CN)8]-4
(v) Fe[EDTA]]–
(vi) [Pd(H2O)2(ONO)2I2]
Answer:
(i) NH3 is a monodentate ligand.
Point of attachment with Cu+2 = 4 x 1 = 4
C.N of Cu+2 = 4
(ii) C2O4-2 is bidentate ligand.
Point of attachment with Fe+3 = 3 x 2 = 6
C.N of Fe+3 = 6
(iii) ‘en’ is a bidentate ligand and Cl– is a monodentate ligand.
Point of attachment with
Pt+2 = 2 x 2 + 2 x 1 = 6
C.N of Pt+2 = 6
(iv) CN– is a monodentate ligand.
Point of attachment with Mo+4 = 8 x 1 = 8
C.N of Mo+4 = 8
(v) EDTA is a hexadentate ligand.
Point of attachment with Fe+2 = 6 x 1 = 6
C.N of Fe+2 = 6
(vi) Point of attachment with
Pd+4 = 2 x 1 + 2 x 1 + 2 x 1 = 6
C.N of Pd+2 = 6
![]()
Question 2.
Calculate the oxidation state, of the central metal atom, in the following:
(i) [CO(NH3)5Cl]+2
(ii) K4[Fe(CN)6]
(iii) [Co(NO2)2(Py)2(NH3)2] NO3
(iv) Ni[CO]4/
(v) [Fe(EDTA)]–
Answer:
(i) x + 5 × (0) – 1 = + 2
x = 2 + 1 = 3
oxidation state of cobalt = +3
(ii) 4 × (+1) + x + 6 (-1) = 0
x = +6 – 4 = +2
oxidation state of Iron = + 2.
(iii) x + 2 (-1) + 2(1) + 2(0) – 1 = 0
x = 2 + 1 = 3
oxidation state of cobalt = +3
(iv) x + 4 (0) = 0 or x = 0:
oxidation State of nickel = 0
(v) x + 1 × x (-4) = -1
x = 4 – 1 = +3
oxidation state of Iron = +3
Question 3.
Give the IUPAC names of the following compounds:
(i) K3[Al(C2O4)3]
(ii) [Pt(NH3)4(NO2)Cl] SO4
(iii) K3[Cr(CN)6]
(iv) [CO(NH3)5ONO]Cl2
(v) [Cr(NH3)5CO3]Cl
(vi) [Cr(NH3)5 (NCS)][ZnCl4]
(vii) K[Au(CN)2]
Answer:
(i) Pottasium trioxalatoalurhinate (III)
(ii) Tetraamminechloridonitroplatinum (IV) sulphate.
(iii) Potassiumhexacyanochromate (III)
(iv) Pentaammine nitritocobalt (III) chloride.
(v) Pentaammine carbonate chromium (III) chloride.
(vi) Pentaammine isothiocyanato chromium (III) chloride.
(vii) Potassium dicyanoaurate (I).
![]()
Question 4.
Write the formula of the following co-ordination compounds.
(i) Tetraammine diaqua cobalt (III) chloride.
(ii) Potassium tetracyano nickelate (II).
(iii) Tris (ethane 1,2, diamine) chromium (III) chloride.
(iv) Amminebromidochloridonitrito-N- platinate (III).
(v) Dichlorido bis (ethane 1, 2, diamine) platinum (IV) nitrate.
(vi) Iron (III) hexacyanoferrate (III).
Answer:
(i) [Co(NH3)4(H2O)2]Cl3
(ii) K2[Ni(CN)4]
(iii) (Cr (en)3]Cl3
(iv) [Pt(NH3)Br Cl (NO2)]‘-
(v) [Pt Cl2 (en)2] (NO2)2
(vi) Fe4(Fe(CN)2]3
Question 5.
Write the IUPAC names of the following
co-ordination compounds:
(i) [Co(NH3)6]Cl3
(ii) [Co(NH3)5Cl]Cl2
(iii) K3[Fe(CN)6]
(iv) K3[Fe (C2O4)3]
(v) K2[Pd Cl4]
(vi) Pt [(NH3)2 Cl (NH2CH3)]Cl.
Answer:
(i) Hexaammine cobalt (III) chloride.
(ii) Pentaammine chloridocobalt (III) chloride.
(iii) Potassium hexacyano ferrate (III).
(iv) Potassium trioxalato ferrate (III).
(v) Potassium tetrachlorido palladate (II).
(vi) Diammine chlorido (methylamine) platinum (II) chloride.
Question 6.
Using IUPAC names, write the formula of the following:
(i) Tetra hydrazozincate (II)
(ii) Hexaammine cobalt (III) sulphate.
(iii) Potassium trioxalato chromate (III)
(iv) Diammine dichlorido platinum (II)
(v) Tetra bromido cuprate (II)
(vi) Pentaamine nitrito-O-cobalt (III)
(vii) Pentaamine nitrito-N-cobalt (III)
Answer:
(i) [Zn(OH)4]-2
(ii) [CO(NH3)6]2SO4
(iii) K3[Cr (C2O4)3]
(iv) [Pt(NH3)2Cl2]
(v) [Cu Br4]-2
(vi) [CO(NH3)5 (ONO)]+2
(vii) [CO(NH3)5NO2]+2
![]()
Question 7.
Specify the oxidation numbers of the metal ions in the following coordination entities.
(i) [CO(H2O) (CN) (en)2]+2
(ii) [Pt Cl4]-2
(iii) [Cr(NH3)3 Cl3]
(iv) [Co Br2 (en)2]+
(v) K3[Fe(CN)6]
Answer:
(i) x + 0 + (-1) + 0 = + 2; x = +3
(ii) x – 4 = – 2 ; or x = +2
(iii) x + 0 + 3(-1) = 0; x = +3
(iv) x – 4 = -2 or x = +2
(v) 3(1) + x + 6(-1) = 0; or x = +3
Question 8.
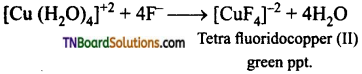
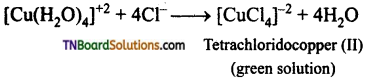
Aqueous copper sulphate (blue in colour) gives (i) a green precipitate with aqueous potassium fluoride and (ii) a bright green solution with aqueous potassium chloride. Explain these experimental results.
Answer:
Aqueous copper sulphate exists as [Cu(H2O)4] SO4. It is a labile complex. The blue colour is due to [Cu(H2O)4]+2 ions.
(i) When KF is added, weak H2O ligands are replaced by F– ligands forming [CuF4]-2 ions which is a green precipitate.
(ii) When KCl is added, Cl– ligands replaced by weak H2O ligands forming [CuCl4]-2 ion which has a bright green colour.
Question 9.
Give two examples for each of the following:
(i) Cationic complex
(ii) Anionic complex
(iii) Neutral complex
Answer:
(i) [Ag(NH3)2]+, [Co(NH3)6]+3
(ii) [Co (CN)6]-3, [Fe(CN)6]-4
(iii) [Ni(CO)4], [CO(NH3)3 Cl3]
Question 10.
What are homoleptic and heteroleptic complexes? Give one example for each.
Answer:
A coordination compound in which the central metal atom / ion is co-ordinated to only one kind of ligand is called homoleptic complex. Examples (Co(NH3)6]+3, [Fe(H2O)6]+3.
A coordination compound in which the central metal atom / ion is coordinated to more than 6ne kind of ligand is called heteroleptic complex. Example [CO(NH3)5Cl]+2, [Pt(NH3)2Cl2].
![]()
Question 11.
Identify the following complexes as homoleptic or heteroleptic complexes.
(i) [Fe(CN)6]-4
(ii) [CO(NH3)4Cl2]+
(iii) [Cr(en)3]+3
(iv) [Ag(NH2)2]+
(v) [Fe(CN)5NO]-3
Answer:
(i) Homoleptic complex ion.
(ii) Heteroleptic complexion.
(iii) Homoleptic complexion.
(iv) Homoleptic complexion.
(v) Heteroleptic complex ion.
Question 12.
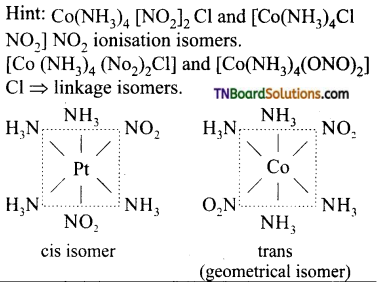
Explain the following giving an example in each case: (i) Linkage isomerism, (ii) Coordination isomerism, (iii) Ionisation isomerism, (iv) Solvate or hydrate isomerism.
Answer:
(i) Linkage isomerism This type of isomers arises when an ambidentate ligand is bonded to the central metal atom/ion through either of its two different donor atoms, eg: In the nitrite ion is bound to the central metal ion Co3+ through a nitrogen atom in one complex,and through oxygen atom in other complex.
[CO(NH3)5(NO2)]2-
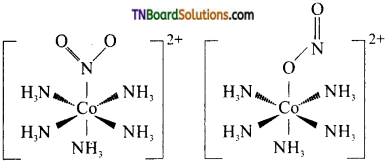
(ii) Coordination isomerism: This type of isomerism arises in coordination compounds having both complex cations and complex anions. The interchange of one or more ligands between the cationic and the anionic coordination entities gives different isomers, eg:
[CO(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [CO(CN)6]
[Cr(NH3)6] [Co(C2O4)3] and [Co(NH3)6]
[Cr(C2O4)3]
[Co(en)3] [Cr(CN)6] and [Cr(en)3] [CO(CN)6]
(iii) Ionisation isomerism: This type of isomerism arises when the coordination compounds give different ions in solution. For example, the complex having the formula [Co(NH3)5 BrSO4] exists in two isomeric forms.
[CO(NH3)3 Br]SO4 and [Co(NH3)5SO4] Br. They give different ions in an aqueous solution.
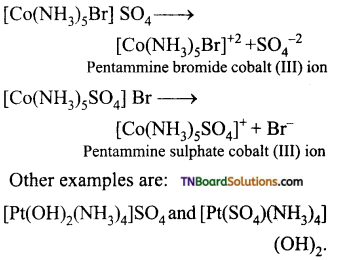
(iv) Solvate or hydrate isomerism: This isomerism arises when free solvent molecules like H20, ammonia or alcohol present in the coordination entity are exchanged with the ligands outside the coordination entity.
eg: [Cr(H2O)6]Cl3, [Cr(H2O)5Cl]Cl2 H2O [Cr(H2O)4Cl2] Cl2H2O.
Question 13.
Give the ionisation isomer of the following and write their IUPAC names.
(i) [Pt(NH3)4 Cl2] Br2
(ii) [CO(NH3)4Cl2] NO2
Answer:
(i) The ionisation isomer of [Pt(NH3)4 Cl2] Br2 is [Pt(NH3)4 Br2] Cl2. Its IUPAC name is tetraammine dibromido platinum (IV) chloride.
(ii) The ionisation isomer of [CO(NH3)4 Cl2] NO2 is [CO(NH3)4Cl.NO2]Cl. Its IUPAC name is tetraammine chlorido nitro cobalt (III) chloride.
![]()
Question 14.
Briefly outline the geometrical isomerism exhibited by square planar and with an example.
Answer:
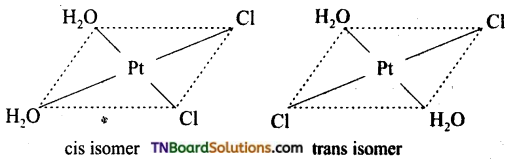
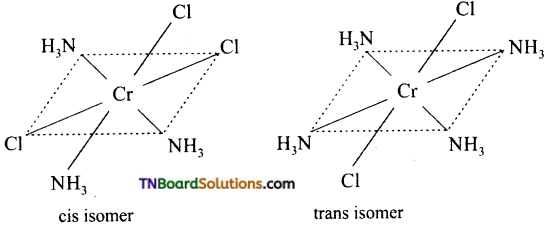
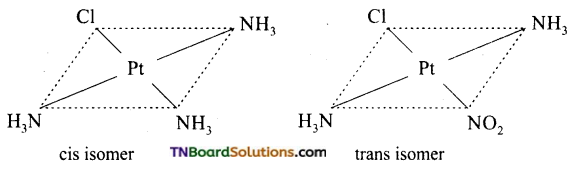
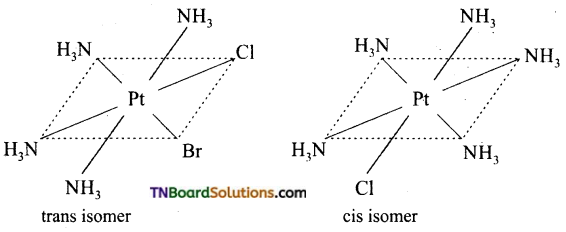
Square planar complexes: In square planar complexes of the form [MA2B2]n± and [MA2BC]n± (where A, B and C are mono dentate ligands and M is the central metal ion/ atom), Similar groups (A or B) present either on same side or on the opposite side of the central metal atom (M) give rise to two different geometrical isomers and they are called, cis and trans isomers respectively. The square planar complex of the type [M(xy)2]n± where xy is a bidentate ligand with two different coordinating atoms also shows cis-trans isomerism. Square planar complex of the form [MABCD]n± also shows geometrical isomerism. In this case, by considering any one of the ligands (A, B, C or D) as a reference, the rest of the ligands can be arranged in three different ways leading to three geometrical isomers.
eg:
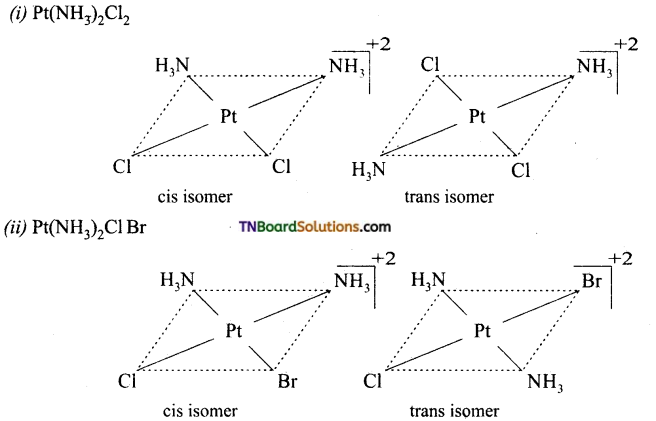
Question 15.
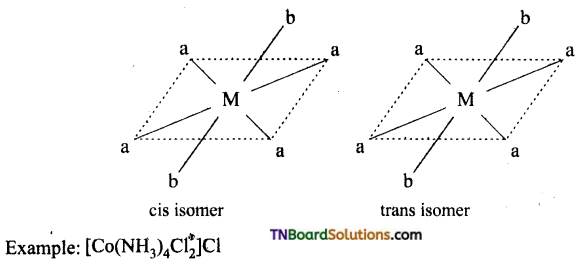
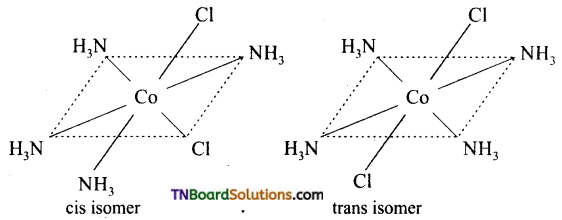
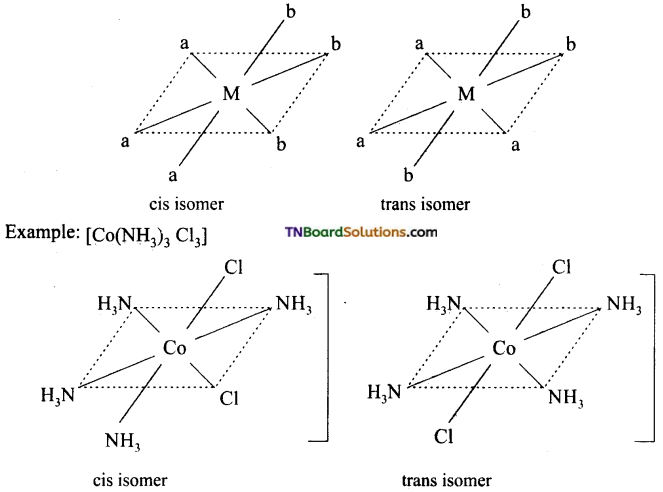
Write the structure of geometrical isomers in octahedral complexes of the type Ma4b2, Ma2b4, Ma4bc, Ma3b3 where a + b are monodentate ligands. Give example for each type.
Answer:
(i) Ma4b2 type:
(ii) Ma3b3 type:
(iii) M(aa3)2b2 or M(aa)2 bc type:
where aa is a symmetrical bidentate ligand, b and c are monodentate ligands, eg: [Co(en)2Cl2]+
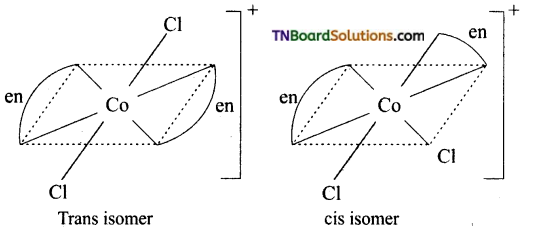
Question 16.
Mention the types of stereoisomerism exhibited by coordination compounds.
Answer:
Coordination compounds exhibit geometrical and is optical isomerism.
![]()
Question 17.
Explain the geometrical isomerism exhibited by the [MA3B3]±n where A and B are monodentate ligands. [OR]
Explain the terms facial and meritorial isomers.
Answer:
Octahedral complex of the type [MA3B3]n± also shows geometrical isomerism. If the three similar ligands (A) are present in the comers of one triangular face of the octahedron and the other three ligands (B) are present in the opposing triangular face, then the isomer is referred as a facial isomer (fac isomer). If the three similar ligands are present around the meridian which is an imaginary semicircle from one apex of the octahedral to the opposite apex as shown in the figure, the isomer is called as a meridional isomer (mer isomer). This is called meridional because each set of ligands can be regarded as lying on a meridian of an octahedron.
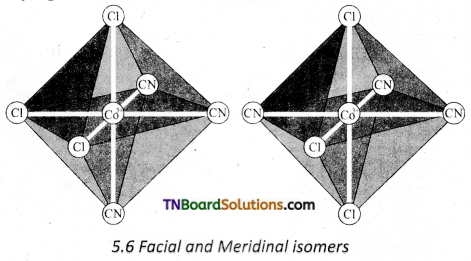
Question 18.
Write the formula of coordination isomers of the following. Write their IUPAC names.
(i) [Pt(NH3)4] [CuCl4]
(ii) [Cr(NH3)6] [Co(C2O4)3]
(iii) [Cr(NH3)6] [Cr(SCN)6]
(iv) Co(en)3[Cr(CN)6]
Answer:
(i) The coordination isomer of [Pt(NH3)4] [CuCl4] is [Cu(NH3)4] [PtCl]4. Its IUPAC name is Tetraammine copper (II) tetrachlorido platinate (II).
(ii) The coordination isomer of [Cr(NH3)6] [Co(C2O4)3] is [CO(NH3)6] [Cr(C2O4)3]. Its IUPAC name is Hexaammine cobalt (III) trioxalato chromate (III).
(iii) The coordination isomer of [Cr(NH3)6] [Cr(SCN)6] is [Cr(NH3)4(SCN)2] [Cr(NH3)2(SCN)4] Tetraammine dithiocyanatochromium (III) diammine tetrathiocyanatochromate (III).
(iv) The coordination isomer of [Co(en)3] [Cr(CN)6] is [Cr(en)3]4 [Co(CN)6], Its ionisation isomer is Tri (ethane 1, 2, diamine) chromium (III) hexacyano cobaltate (III).
Question 19.
Discuss the bonding in metal carbonyls.
Answer:
The metal-carbon bond in metal carbonyls have both σ and π bond character. The metal – carbon sigma bond is formed by the donation of lone pair of electrons from the carbonyl carbon into a vacant orbital of the metal. The metal- carbon pi bond is formed by the donation of a pair of electrons from a filled d orbital of the metal to the vacant antibonding pi molecular orbital of carbon monoxide. The metal ligand bonding causes a synergic effect which strengthens the bond between Co and the metal.
![]()
Question 20.
Indicate the type of isomerism exhibited by the following complexes and draw structures for these isomers.
(i) KCr (H2O)2(C2O4)3
(ii) [Co(en)3]Cl3
(iii) [ CO(NH3)5(NO2)(NO3)2]
(iv) [Pt (NH3) (H2O)Cl2]
Answer:
(i) Both show geometrical (cis-trans) isomerism. Optical isomerism is shown by cis form.
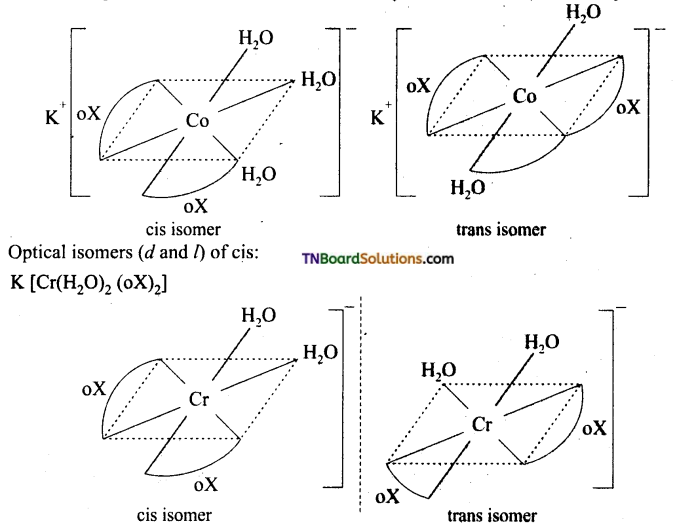
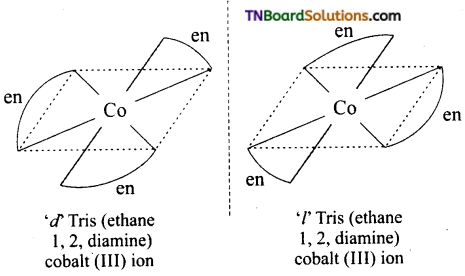
(ii) Two optical isomers can exist. [Co(en)3]+
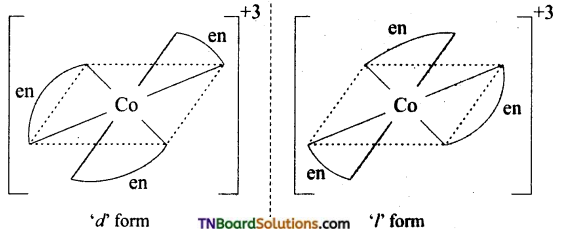
(iii) There are 10 possible (geometrically ionisation and linkage) isomers.
Ionisation isomers:

(iv) Geometrical (cis-trans) isomerism can exist.
Question 21.
Give evidence to show that [CO(NH3)5Cl]SO4 and [Co(NH3)5 SO4]Cl are ionisation isomers.
Answer:
The ionisation isomers dissolve in water to yield different ions and thus react differently with different reagents.
![]()
A white precipitate is than obtained.
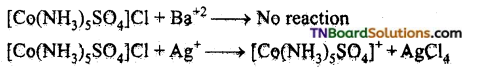
Question 22.
Draw the structure of optical isomers of (i) PtCl2 (en)2, (ii) [Cr(NH3)2 Cl2 (en)2]+
Answer:
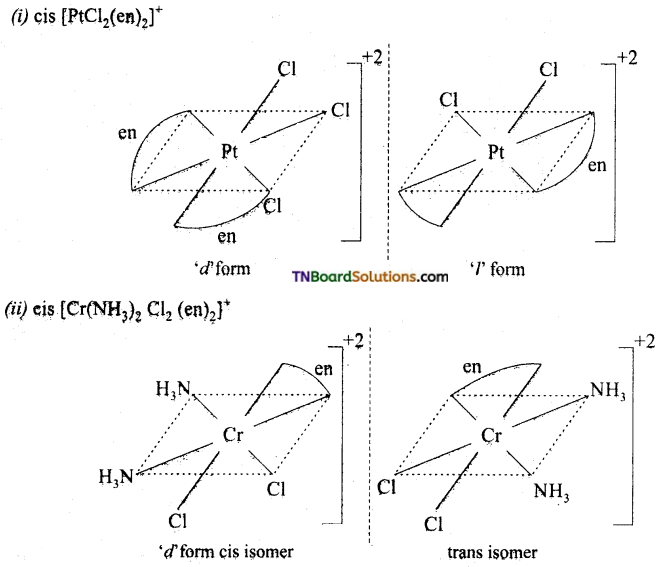
Question 23.
Write all the geometrical isomers of [Pt (NH3) BrCl Py] and how many of these exhibit optical isomerism?
Answer:
Three isomers are possible.
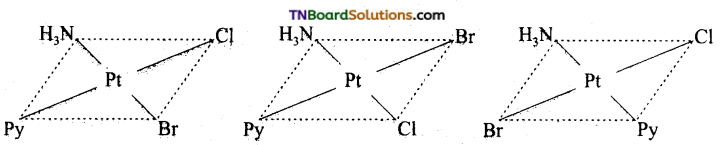
Isomers of this type do not show optical isomerism. Optical isomerism rarely occurs in square planar or tetrahedral complexes and that too when they contain asymmetrical chelating ligand.
![]()
Question 24.
Draw the structure of all the isomers (geometrical and optical) of (i) [CO(NH3)Cl (en)2]+2 (ii) [Co(NH3)2Cl2(en)]+
Answer:
(i)
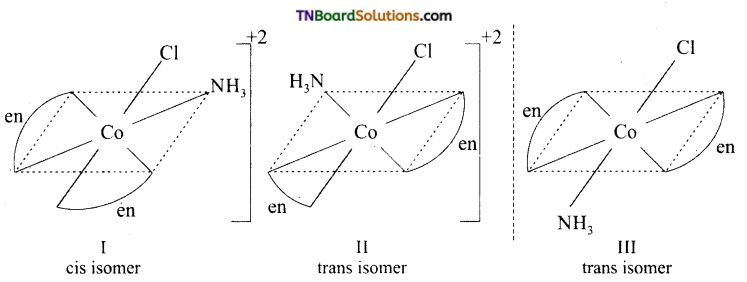
I and II are optical isomers.
II and III are geometrical isomers.
(ii)
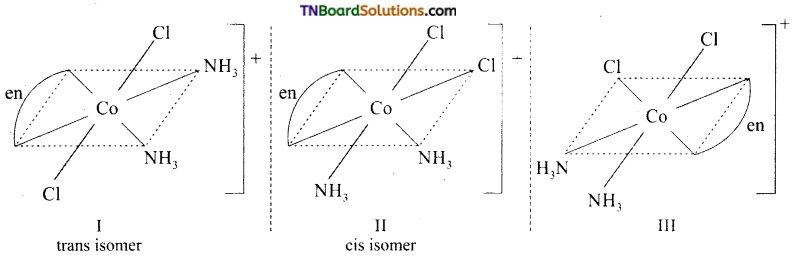
I and II are geometrical isomers.
II and III are optical isomers.
Question 25.
Draw the structure of geometrical isomers of [Fe(NH3)2(CN)4]–.
Answer:
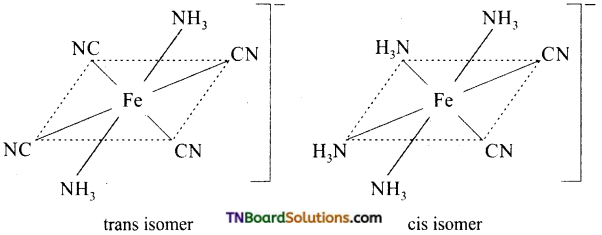
Question 26.
Out of the following two coordination entities which is optically active? (i) cis [CrCl3(oX)2]-3(ii) trans [Cr Cl2 (oX)2]-3 The two entities are represented as follows:
Answer:
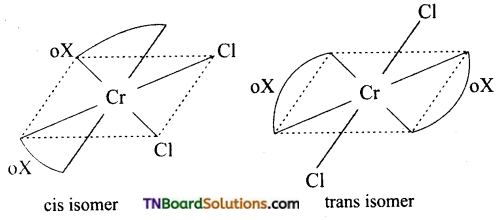
Of these cis form is optically active.
Question 27.
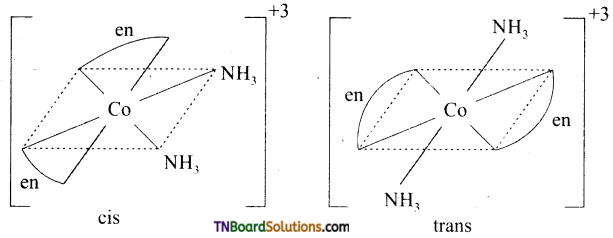
Write the structures of isomers, if any and write the names of the following complexes. (i) [Cr (NH3)4 Cl2]+, (ii) [Co(en)3]+
Answer:
(i) Geometrical isomers of [Cr(NH3)4Cl2]+
(ii) Optical isomers of OX [Co(en)3]+3
Question 28.
Platinum (II) forms’square planar complexes and platinum (IV) gives octahedral complexes.. How many geometrical isomers are possible for each of the following. Draw their structures. (a) [Pt(NH3)3Cl]+, (b) [Pt(NH3)Cl5]–, (c) [Pt(NH3)2ClNO2], (d) [Pt(NH3)4Cl Br]+2.
Answer:
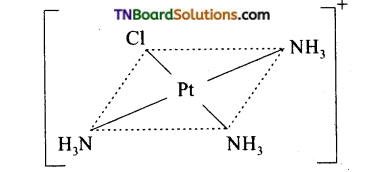
(a) No isomer is possible for a square planar complex MA3B.
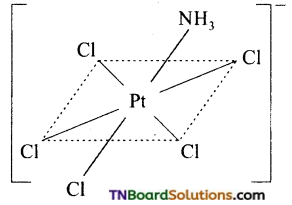
(b) No isomer is possible for an octahedral complex of the type MAB5.
(c) cis-trans isomers are possible for a square planar complex MA2BC.
(d) cis-trans isomers are possible for an octahedral complex of the type MA2BC.
![]()
Question 29.
Explain the term (i) inner orbital complex and (ii) outer orbital complex with an example for each.
Answer:
(i) Bonding in complexes are explained in terms of hybridisation. If the hybridisation of central metal atom/ion involves the use of (n-1) d, ns and np orbitals, then d2sp3 hybridisation is possible for octahedral complexes. Complexes formed by d2sp3 hybridisation of the central metal atom or ion is known as inner orbital complex. [Fe(CN)6]-4, [Fe(CN)6]-3, [Cr(NH3)6]+3 are examples of inner orbital complexes.
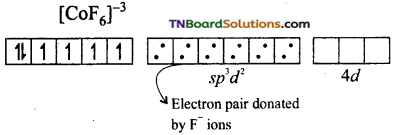
(ii) If the hybridisation of the metal ion involved the ns, np and nd orbitals and for octahedral complexes sp3d2 hybridisation involved, the complex formed is known as outer orbital complex. [CoF6]-3 is an example for outer orbital complex.
Question 30.
[Co(CN)6]-3 and [CoF6]-3 are both octahedral complexes. Then what is the difference between the two?
Answer:
In both complexes, Co is in +3 oxidation state. It has 6 electrons in the ‘d’ subshell (d6).

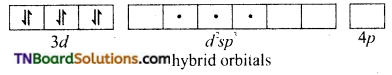
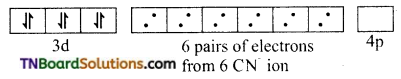
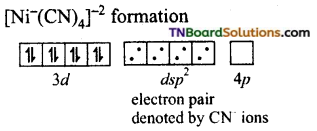
In the formation of [Co(CN)6]–, the ‘d’ electrons in 3d level gets paired leaving behind two 3d orbital vacant.

The Co+3 ion makes use of two 3d orbitals, one 4s and two 4p orbitals for hybridisation. d2 sp3 hybridisation and two 4p orbitals for hybridisation.
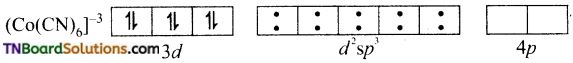
The CN– ion donates the electron pairs to the d2spi hybrid orbitals and form six covalent bond. Thus [(CO(CN)6]-3 is an inner orbital or low spin complex.
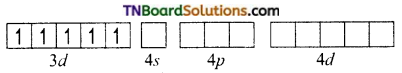
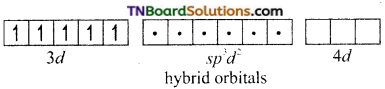
On the other hand, in the formation of [CoF6]-3 the Co+3 ion makes use of outer 4s, 4p and 4d orbitals for hybridisation.

In the presence of F– ion, electron pairing does not occur in 3d orbitals, and Co+3 ion makes use of one 4s, three 4p and two 4d orbitals for hybridisation.

The six fluoride ion donates a pair of electrons to the six sp3d2 hybrid orbitals and form six-coordinate covalent bonds.
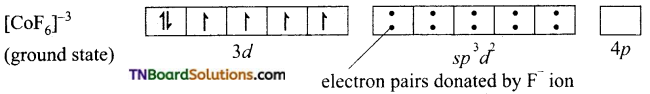
Since outer orbitals are used in the formation it is an outer orbital complex. Since more unpaired electrons are present it is also known as high spin complex.
Question 31.
Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory.
(i) [Fe(CN)6]-4
(ii) [FeF6]-3
(iii) [Co(C2O4)3]-3
(iv) [NiCl4]-2
Answer:
(i) [Fe(CN)6]-4: d2sp3 hybridisation octahedral: diamagnetic.
In this complex, the oxidation state of iron is +2.
Electronic configuration of Fe = [Ar]3d46s2
Electronic configuration of Fe+2 = [Ar]3d6
To accommodate six pairs of electrons from six cyanide ions, the iron (II) must make available six empty orbitals. This can be achieved by the d2sp3 hybridisation.

In the presence of CN– ion, a strong ligand, the electrons in 3d subshell gets paired and two of the 3d one 4s and three 4p orbitals hybridise to produce six d2sp3 hybrid orbitals.
Fe+3 (d6) excited state.
Thus, the six pairs of electrons from six cyanide ion occupy the six hybridised orbitals of iron (II) ion. [Fe(CN)6]-4
Since, there are no unpaired electron present, it is diamagnetic and its geometry is octahedral.
(ii) [FeF6]-3: This complex is high spin or octahedral, since the central atom Fe(II) utilises nd orbitals for hybridisation. It is an octahedral complex involving sp3d2 hybridisation. Each orbital accommodates a lone pair of electrons from six fluoride ions as shown below. [Fe+3(d5) ground state]
In the presence of F– ions (weak ligand) the electrons in 3d orbitals do not get paired and the iron (II) utilises ns, np and nd orbitals for hybridisation. Thus six sp3d2 hybrid orbitals are formed.
[Fe+3 excited state]
The six pairs of electrons from F ions accommodate the six vacant spid1 hybrid orbitals
[FeF6]-3
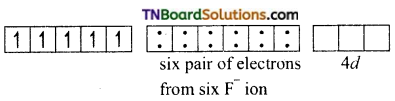
Thus, the complex is outer orbital complex, octahedral and paramagnetic.
(iii) [Co(C2O4)3]-3: d2sp3 hybridisation diamagnetic.
The cobalt ion is in +3 oxidation state. It has six electrons in d subshell (d6).
[Co+3(d6)]
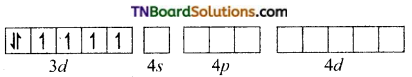
Oxalate ion being a relatively strong ligand pairs of the electrons in ‘d’ subshell, learning two of the ‘d’ orbital for hybridisation. Thus Co+3 ion under goes d2sp3 hybridisation.
Co+3(ground state)
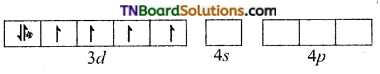
The three oxalate anions (bidentate) donate two pairs of electrons to two of the hybrid orbitals.
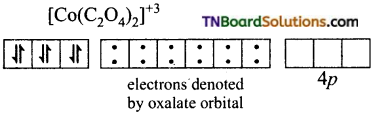
Thus [Co(C2O4)3]-3 is an inner orbital, octahedral, complex and diamagnetic.
(iv) [NiCl4]-2: In this complex Ni is in +2 oxidation state and has d8 configuration.
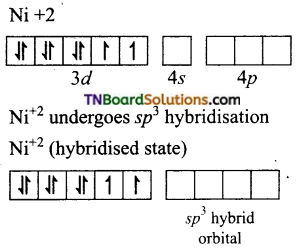
The chloride ions donate pair of electrons to each of the four sp3 hybrid orbitals.
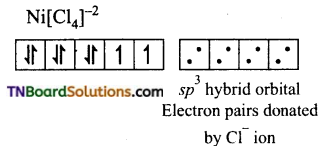
Thus, the complex is tetrahedral and paramagnetic.
Question 32.
[NiCl4]-2 is paramagnetic while Ni(CO)4 is diamagnetic though both are tetrahedral why?
Answer:
In Ni(Co)4, Ni is in the zero oxidation state whereas in [NiCl4]-2, it is in +2 oxidation state. In the presence of a CO being a strong ligand, the unpaired ‘d’ electrons of Ni pair up but Cl– being a weak ligand it is unable to pair up the unpaired electrons. Hence [NiCl4]-2 is paramagnetic where as Ni(CO)4 is diamagnetic.
![]()
Question 33.
Explain [Co(NH3)6]+3 is an inner orbital complex whereas [Ni(NH3)6]+2 is an outer orbital complex.
Answer:
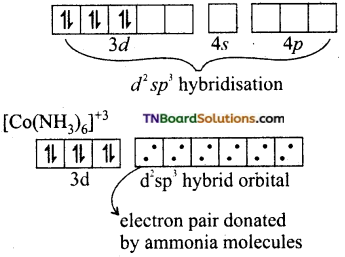
In the presence of NH3, the 3d electrons pair up learning two ‘d’ orbitals empty to be involved in d2sp3 hybridisation forming inner orbital complex.
In [Ni(NH3)6]+2, Ni is in +2 oxidation state and has d8 configuration. The hybridisation involved in sp3d2 and hence, it forms an outer orbital complex.
Question 34.
[Cr(NH3)6]+3 is paramagnetic while [Ni(CN)4]-2 is diamagnetic explain why?
Answer:
(i) Formation of [Cr(NH3)6]+3:
The oxidation state of chromium in [Cr(NH3)6]+3 ion is +3. The electronic configuration of chromium is [Ar] 3d5 4s1. The hybridisation scheme is shown below:
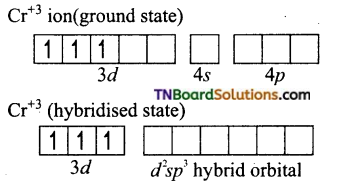
Ammonia being a weak ligand is unable to pair of up the electrons of Cr+3 and hence Cr+3 undergoes d2sp3 hybridisation.
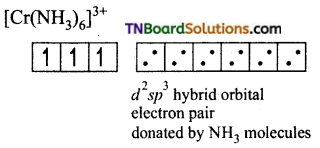
Thus the complex is inner orbital, octahedral complex and paramagnetic because of the presence of three unpaired electrons.
(ii) Formation of [Ni(CN)4]-2: In the square planar complexes, the hybridisation involved is dsp2. In [Ni(CN)4]-2, nickel is in +2 oxidation state and has the electronic configuration of d8.

CN– ion being a strong ligand, pairs up the 3d electrons leaving one 3d orbital vacant that is used for dsp2 hybridisation.
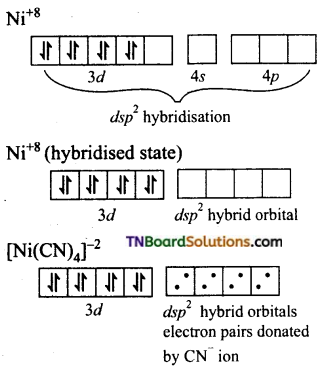
Thus the complex is diamagnetic as there are no impaired electron.
Question 35.
A solution of [Ni(H2O)6]+2 is green but a solution of [Ni(CN)4]-2 is colourless explain.
Answer:
In [Ni(H2O)6]+3, Ni is in +2 oxidation state with configuration d8. i.e., it has 2 unpaired electrons which do not pair in the presence of weak H2O ligand. Hence d-d transition is possible and so it is coloured. The d-d transition absorbs red light and the complimentary light emitted is green.
In case of [Ni(CN)4]+2, Ni is in +2 oxidation state with d8 configuration. But in the presence of strong CN– ion as ligand, the two unpaired electrons in 3d orbitals are paired up. Hence there are no unpaired electrdhs present and so it is colourless.
![]()
Question 36.
[Fe(CN)6]-4 and [Fe(H2O)6]+2 are of different colours in dilute solution why?
Answer:
In both complexes, Fe is in +2 oxidation state with d6 configuration. In the presence of a weak ligand, they do not pair up. In the presence of strong CN– ion, they paired up learning no unpaired electrons. Due to the difference in the number of unpaired electrons they have different colours.
Question 37.
Write down the IUPAC name of each of the following complexes and indicate the oxidation state, electronic configuration and coordination number of the central metal ion. Also, give the stereochemistry and magnetic moment of each complex.
(i) K4[Cr(H2O)2(C2O4)2]
(ii) [CrCl3(Py3)]
(iii) K2[Mn(CN)4]
(iv) [CO(NH3)5Cl]Cl2
(v) CS[FeCl4]
Answer:
(i) Potassiumdiaquaoxalatochromate (III) hydrate.
Coordination number = 6
Shape: Octahedral
Oxidation state of Cr = x + 0 + 2 (- 2) = -1 or x = +3

(ii) Trischloridopyridinechromium (III)
Coordination number of Cr = 6
Shape: Octahedral
Oxidation state of Cr = x – 3 + 0 + 0 = 0 or x = +3
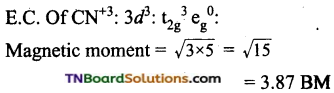
(iii) Potassiumhexacyanomanganate (II)
Coordination number of Mn = 6.
Shape: Octahedral
Oxidation state of Mn = x – 6 = – 4 (or) x = +2

(iv) Pentaammine chloridocobalt (III) chloride.
Coordination number of Co = 6.
Shape: Octahedral
Oxidation state of Cr = x + 0 – 1 = +2 or x = +3
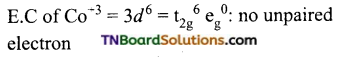
Magnetic moment = 0
(v) Caesiumtetrachloridoferrate (III).
Coordination number of Fe = 4.
Shape: Tetrahedral
Oxidation number of Fe = x – 4 = -1 or x = +3

Question 38.
Calculate the magnetic moment of the following coordination entities.
(i) [Cr(H2O)6]+3,
(ii) [Fe(H2O)6]+3,
(iii) [Zn(H2O)6]+2
Answer:
The oxidation states are (i) Cr(III); (ii) Fe(II) and (iii) Zn(II)
(i) E.C of Cr+3 = 3d3:
no ofunpaired electrons = 3
Magnetic moment = \(\sqrt{3(3+2)}\) = √15
(ii) E.C of Fe+2 = 3d6:
no of unpaired electrons = 4
Magnetic moment = \(\sqrt{4(4+2)}\) = √24
(iii) E. C of Zn+2 = 3d10:
no of unpaired electrons = 0
Magnetic moment = 0
![]()
Question 39.
What will be the correct order for the wavelength of absorption in the visible region of the following:
[Ni(NO2)6]-4, [Ni(NH3)6]+2, [Ni(H2O)6]+2
Answer:
As the metal ion is fixed the increasing field strength (CFSE values) of the ligands from the spectrochemical series are in the order.
H2O<NH3<NO2–
Thus the energies absorbed for excitation will be in the same order
[Ni(H2O)6]+2 < [Ni(NH3)6]+2 < [Ni(H2O)6]+2
As, E = \(\frac{h c}{\lambda}\), the wavelength absorbed will be in the opposite order.
Question 40.
Give the number of unpaired electrons in the following complex ions: (i) [FeF6]-4, (ii) [Fe(CN)6]-4
Answer:
(i) In [FeF6]-4, Fe is in +2 oxidation state. It has d6 configuration. As F– is a weak ligand, the electrons in 3d orbital are not paired.
i-e., ![]()
Hence, it has 4 unpaired electrons.
(ii) In [Fe(CN)6]-4 also, Fe is in +2 oxidation state and it has d6 configuration. Because CN– ion is a strong ligand, the electrons in 3d orbital of Fe+2 are paired and so there are no unpaired electrons.
Question 41.
Explain as how two complexes [Ni(CN)4]-2 and [Ni(CO)4] have different structures but do not differ in their magnetic behaviour. (Ni = 28)
Answer:
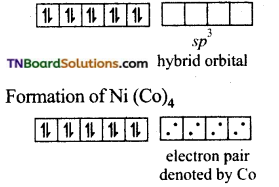
(i) In Ni(CO)4, nickel is in zero oxidation state. (Ni+2 = 3d8 4s2)
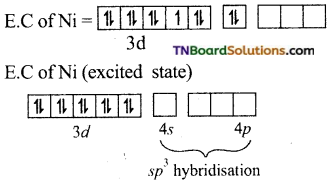
E.C of Ni in hybridised state
It is diamagnetic and has a tetrahedral geometry.
(ii) In [Ni(CN)4]-2, Ni is in +2 oxidation state. Electronic configuration of Ni+2 ion is 3d8.
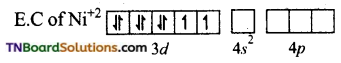
CN– ion being a strong liquid and in its presence, the electrons in 3d orbital gets paired leaving are 3d orbital for hybridisation. Thus, it undergoes dsp2 hybridisation.
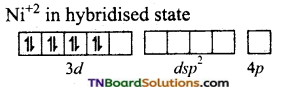
The CN– ions denote a pair of electrons to the valent dsp2 hybrid orbitals. Thus, four Ni– CN bonds are formed.
Thus the geometry is square planar and the complex is diamagnetic.
Question 42.
Using valence bond theory explain [Co(NH3)6]+3 in relation to the complex given below:
(i) type of hybridisation [Co(NH3)6]+3 : Co+3 = d6 configuration
(ii) inner or outer orbital complex
(iii) magnetic behaviour
(iv) spin only magnetic moment value.
Answer:
(i) In the presence of ammonia ligand, the electrons in 3d level gets paired up leaving two of the 3d orbitals for hybridisation. Hence, the type of hybridisation is d 2sp3
(ii) Since inner ‘d’ orbitals are used for hybridisation, it is an inner orbital complex.
(iii) The complex is diamagnetic as there are no unpaired electrons.
(iv) Since, there are no unpaired electrons the magnetic moment is zero.
![]()
Question 43.
Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory.
(i) [FeCN6]-4
(ii) [FeF6]2-
(iii) [Co(C2O4)3]-3
(iv) [COF6]-3
Answer:
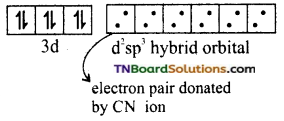
(i) Oxidation state of Fe in the complex is +2. Its electronic configuration is [Ar] 3d6. Iron undergoes d2sp3 hybridisation giving octahedral, inner orbital complex.
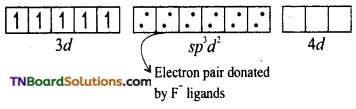
(ii) Oxidation state of iron in the complex is +3. Its electronic configuration is [Ar] 3d5. In the presence of F– (weak ligand), the 3d electrons do not get paired. Hence it undergoes sp3d2 hybridisation and forms an outer orbital, octahedral complex.
(iii) The oxidation state of cobalt in the complex is +3. Its electronic configuration is [Ar] 3d6. Since oxalate ion is relatively a strong ligand, the 3d electrons get paired up leaving two of 3d orbital for hybridisation. Thus, cobalt undergoes d2sp3 hybridisation giving octahedral, inner orbital complex.
(iv) Oxidation state of cobalt in the complex is +3. Its electronic configuration is [Ar] 3d6. The F ion being a weak ligand is unable to pair up the electrons in 3d level. It undergoes sp3d2 hybridisation giving an octahedral, outer orbital complex.
Question 44.
What are crystal fields?
Answer:
The ligands especially anionic (or polar neutral ligands) has around them negatively charged field because of which they are called crystal fields.
Question 45.
What do you understand by the term crystal field splitting?
Answer:
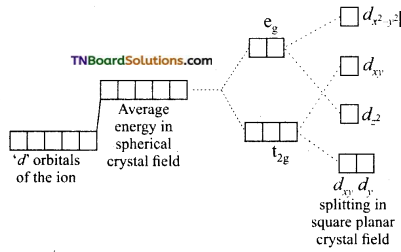
When the ligands approach the central metal ion, the electrons in the ‘d’ orbital of central metal ion will be repelled by the lone pairs of the ligands. Because of these interactions, the degeneracy of ‘d’ orbitals of the metal ion is lost and they split into two sets of orbitals having different energies. This is known as crystal field splitting. The extent of crystal field splitting depends on the nature of the ligand.
Question 46.
Briefly discuss the crystal fieid splitting in octahedral complexes.
Answer:
As a result of repulsive forces between the ‘d’ electrons of the metal ion and that of ligands (negative or polar neutral), the degeneracy of the ‘d’ orbitals is lost. Since the two lobes of two eg{dx2-y2, dz2) orbitals lie in the path of approaching ligands these orbitals experience greater forces of repulsion than those of t2g (dxy, dyz and dxz) orbitals is decreased.
Thus, an energy difference exists between two sets of orbitals. This energy difference is called crystal field splitting energy and is represented by Δo. (the subscript ‘o’ stands for octahedral). It measures the crystal field strength of the ligands. The crystal field splitting occurs in such a way that the average energy of the ‘d’ orbitals do not change.
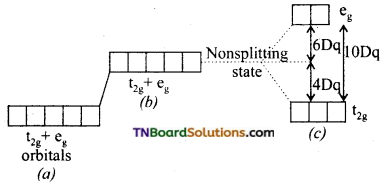
(a) Five degenerate ‘d’ orbitals of the free metal ion.
(b) Hypothetical degenerate ‘GP orbitals at higher energy levels under spherically symmetrical ligand field.
(c) Splitting of ‘d’ orbitals under the influence of ligands.
Thus, three orbitals lie at an energy i.e., 2/5 Δo below the average ‘d’ orbital energy and two ‘d’ orbitals lie at an energy 3/5 Δo above the average energy. The energy gap between t2g and eg sets also denoted by 10Dq.
The energy of t2g orbitals is 4Dq less than that of hypothetical degenerate ‘d’ orbitals and that of eg orbitals is 6Dq above that of hypothetical degenerate ‘d’ orbitals: Thus, t2g set loses energy equal to 0.4 Δo or 4 Dq white eg sets gain energy equal to 0.6 Δo or 6 Dq.
![]()
Question 47.
For the complex
[Fe(en)2Cl2]Cl (en = ethylene diamine), identify The complex is [Fe(en)2Cl2]Cl.
(i) The oxidation number of Fe.
(ii) The hybrid orbital and shape of the complex.
(iii) The magnetic behaviour of the complex.
(iv) The number of geometrical isomers.
(v) Whether there is an optical isomer also.
(vi) The name of the complex (At. No of Fe 26)
Answer:
(i) Oxidation number of Fe
= x + 2 x 0 + 2(-1)
= +1
x – 2 = +1
⇒ x = 3
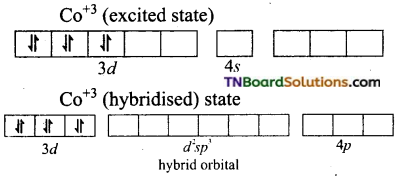
(ii) Orbitals in Fe+3(d5)
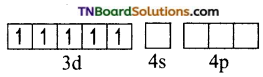
In the presence of a strong ligand (en), the electrons in 3d orbital get paired.
Fe+3 excited state
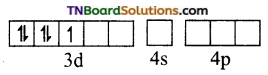
Thus, Fe+3 ion undergoes d2sp3 hybridisation therefore the shape is octahedral.
(iii) The complex is paramagnetic due to the presence of one unpaired electron.
(iv) It exhibits cis-trans isomerism
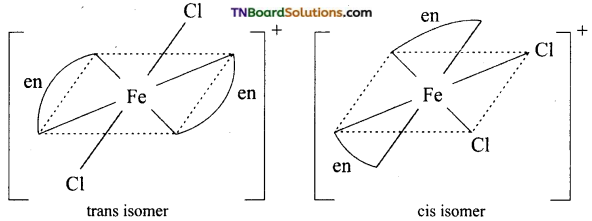
(v) Cis isomer will show optical isomerism.
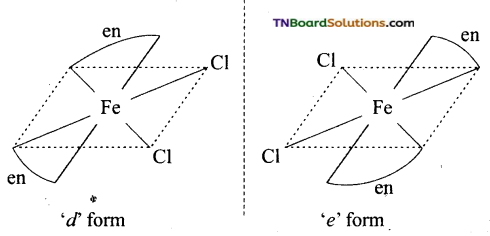
(vi) Dichlorido bis(ethane 1, 2, diamine) iron, (III) chloride.
Question 48.
Compare the following complexes with respect to their shape, magnetic behaviour and the hybrid orbitals involved.
(i) [CoF4]-2, (ii) [Cr(H2O)2(C2O4)2]–, (iii) [Ni(Co)4], (At No: Co = 27, Cr = 24, Ni = 28).
Answer:
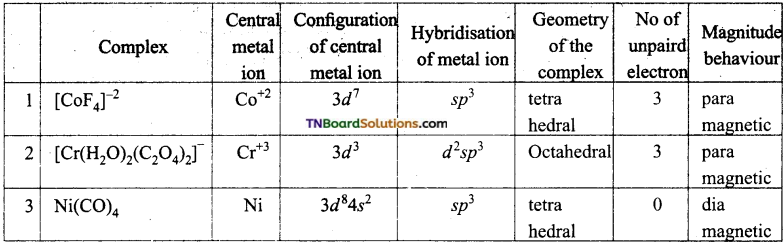
Question 49.
Briefly discuss the crystal field splitting in tetrahedral complexes.
Answer:
The three orbitals i.e., t2g orbitals are close to approaching ligands. As a result of this, the t2g electrons suffer more repulsions than eg electrons. The energy of t2g orbitals increases more than eg orbitals. The splitting is shown below:
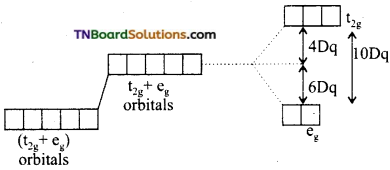
The energy gap between two sets of orbitals is designated as Δt. It is observed that Δt, is considerably less than Δo. It has been found that.
Δt = \(\frac{4}{9}\) Δo
Question 50.
Briefly outline crystal field splitting in square planar complexes.
Answer:
As the lobes of dx2-y2 point towards the ligands, this orbital has the highest energy. The lobes of dxy orbital lie between the ligands but are coplanar with them this orbital is the next highest energy. The lobes of dz2 orbital point out of the plane of the complex but the belt around the centre of the orbital (which contain 1/3 of the electron density) lies in the plane. Therefore dz2 orbital is the next highest in energy. The lobes of dx2 and dy2 orbitals point out of the plane of the complex. Hence, they are least affected by the electrostatic field of the ligands. They are degenerate and lowest in energy.
![]()
Question 51.
What is crystal field splitting energy? How does the magnitude of Δo decide the actual configuration of ‘d’ orbitals in a coordination entity?
Answer:
When ligands approach a transition metal ion, the ‘rf orbital splits into two sets one with lower energy and the other with higher energy. The difference in energy between two sets (t2g and eg) of orbitals is called crystal field splitting energy Δo for an octahedral field.
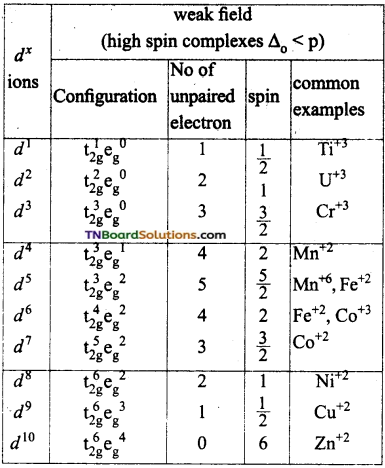
If Δo < p (pairing energy), the fourth electron enters one of the eg orbitals giving the configuration t2g3 eg1, thereby forming high spin complexes. Such ligands for which Δo < p are called weak field ligands.
If Δo > p, the fourth electron pair up in one of the t2g orbital giving the configuration t2g4 eg0, thus forming low spin complexes. Such ligands for which Δo > p are called strong field ligands.
Question 52.
What is spectrochemical series? Explain the difference between a weak field ligand and a strong field ligand.
Answer:
The arrangement of ligands in order of their increasing field strength i.e increasing crystal field splitting energy (CFSE) values is called spectrochemical series.
The ligand with a small value of Δo CFSE (Δo) is called weak field ligands whereas those with a large value of CFSE(Δo) are called strong field ligands.
Question 53.
How does the oxidation state of a metal ion influence or affect the crystal field splitting energy?
Answer:
Generally higher the oxidation state of the metal, greater is the crystal field splitting. For example most of the cobalt (II) complexes have low value of Δ whereas all cobalt (III) complexes have high value of Δ.
Question 54.
Briefly explain the distribution of ‘d’ electrons in t2g and eg orbitals in octahedral complexes in a weak ligand field.
Answer:
Under the influence of weak ligands the energy difference, Δo between t2g and eg sets is relatively small and hence all the five ‘d’ orbitals may be supposed to be degenerate. The distribution of electrons in t2g and eg sets occurs according to Hund’s rule.
When the ligands are weak, the first three electrons numbered as 1,2,3 go to t2g set those numbered 4, 5 go to eg set, those numbered 6, 7, 8 go to t2g set and the remaining two electrons numbered 9 and 10 will occupy eg set. i.e., 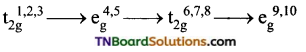
In complexes of weak ligands, Δo is less than P(P is called pairing energy which is the energy required to pair two electrons in the same orbital). Δo is the octahedral crystal field splitting energy, tends to force as many electrons into t2g set while P tends to prevent the electrons to pair up in the t2g level.
Question 55.
The Hexa aqua manganese (II) ions contain five unpaired electrons while hexacyano ions contain only one unpaired electron. Explain using crystal field theory.
Answer:
Mn is in +2 oxidation state and has 3d5 configuration. H2O is a weak ligand. In the presence of water molecules, the distribution of electrons is t2g3 eg2 i.e., all the electrons are unpaired.
In the other hand CN– ion is a strong ligand. The distribution of electrons is t2g5 eg0.
i.e.,  and it has one unpaired electron.
and it has one unpaired electron.
Question 56.
Why do compounds having similar geometry have a different magnetic moment?
Answer:
It is due to the presence of strong and weak ligands in complexes. If GFSE is high, the complex will show a low value of the magnetic moment and vice versa. eS: [CoF6]-3 and [CO(NH3)6]+3, the former is paramagnetic and the latter is diamagnetic.
![]()
Question 57.
Briefly explain how the electrons are distributed between t2g and eg sets in an octahedral complex in the presence of a strong field ligand.
Answer:
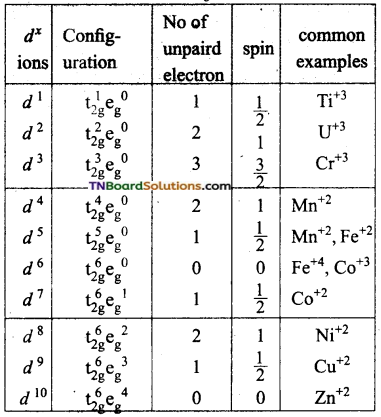
Under the influence of strong ligands, the energy difference between t2g and eg sets is relatively high and thus the distribution of ‘d’ electrons in t2g and eg sets does not obey Hund’s rule. The first electrons numbers 1, 2, 3, 4, 5, 6 will go to t2g set remaining four electrons numbered 7, 8, 9, 10 will go to eg set.
![]()
For these complexes, Δo is greater than p.
Question 58.
Using crystal field theory, draw an energy level diagram of the central metal atom/ion and determine the magnetic moment value of the following.
(i) [COF6]-3,
(ii) [Co(H2O)6]+2,
(iii) [Cu(CN)6]-3
Answer:
(i) F– is a weak field ligand and cobalt is in a +3 oxidation state. It has d6 configuration. The electron distribution is t2g4 eg2.
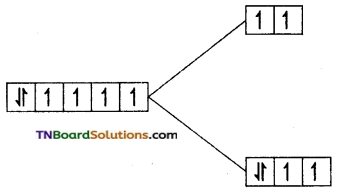
i.e., Number of unpaired electron = 4
Magnetic moment = \(\sqrt{4(4+2)}\) = √24
= 4.9 BM
(ii) In this complex cobalt is in +2 oxidation state. Its electronic configuration is d1. Since water is a weak ligand, electron filling follows Hund’s rule, i.e., t2g5 eg2.
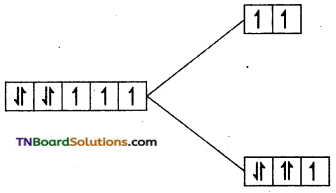
i.e., Number of unpaired electron = 3 Magnetic moment = \(\sqrt{3(3+2)}\) = √15
= 3.87BM
(iii) In this complex cobalt is in +3(d6) oxidation state and CN– ion a strong ligand. Electron filling is not obeyed by Hund’s rule. All the six electrons get paired in t2g set.
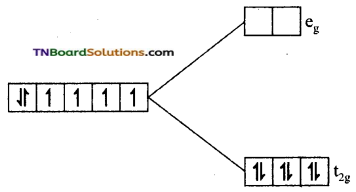
Since, there is no unpaired electron the complex is diamagnetic.
Question 59.
In the basis of crystal field theory explain why cobalt (III) forms a paramagnetic octahedral complex with weak field ligands whereas it forms a diamagnetic octahedral complex with strong field ligands.
Answer:
With weak field ligand, Δo < P, the electronic configuration (of cobalt (III)) i.e., d6 will be t2g4 eg2 and it has 4 unpaired electrons and is paramagnetic. With strong field ligands, Δo > P, the electron distribution is t2g6 eg0. It has no unpaired electron and hence diamagnetic.
![]()
Question 60.
Differentiate between weak field and strong field coordination entity.
Answer:
| Weak field coordination entity | Strong field coordination entity |
| They are formed when the crystal field stabilisation energy (CFSE) in octahedral complexes is less than the energy required for electron pairing in a single orbital (P). | They are formed when crystal field stabilisation energy (CFSE) Δo is greater than pairing energy (P). |
| They are also called high spin complexes. | They are also called low spin complexes. |
| They are mostly paramagnetic. | They are mostly diamagnetic or less paramagnetic than weak field. |
| Never formed by CN– ions. | Formed by CN– like ligands. |
Question 61.
State for a d6 configuration, how the actual configuration of the split ‘d’ orbitals in an octahedral field is decided by relative values of Δo and P.
Answer:
For d6 configuration, three electrons will first enter t2g. Now, if P < Δo electrons will pair up in t2g orbitals giving the configuration t2g6 eg0. If Δo< P, two electrons will first enter into eg orbitals and one will pair up in t2g, giving the configuration t2g4 eg2.
Question 62.
Arrange the following ligands in the increasing order of crystal field splitting power.
Answer:
H2O, OH–, Cl–, F–, CN–
Cl– < F– < OH– < H2O < CN–
Question 63.
Using crystal field theory, show energy level diagrams, write the electronic configuration of the central metal atom/ion and determine the magnetic moment value in the following. (i) [FeF6]-3, (ii) [Fe(H2O)6]+2,
(iii) [Fe(CN)6]-4
Answer:
(i) In this complex, Fe is +3 oxidation state and Fe+3 has 3d5 configuration. Since F– is a weak field ligand, electron filling in t2g and eg sets follow Hund’s rule.
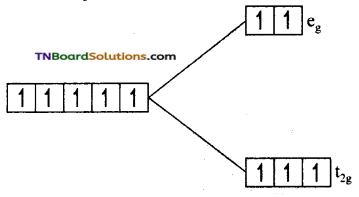
i.e., t2g4 eg2. The number of unpaired electron is 5. The magnetic moment value is \(\sqrt{5(5+2)}\) = √35 = 5.92 BM.
(ii) In [Fe(H2O)6]+2, Fe is in +2 oxidation state and has d6 configuration. Since water is a weak ligand electron distribution in t2g and eg orbitals take place according to Hund’s rule.
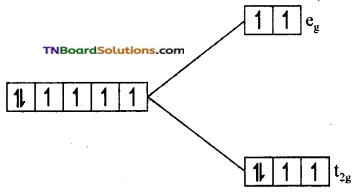
i.e., t2g4 eg2. The number of unpaired electrons is 4. Its magnetic moment value is \(\sqrt{4(4+2)}\) = √24 = 4.9 BM.
(iii) In [Fe(CN)6]-4, Fe is in +2 oxidation state and has 3d6 configuration. As CN is a strong ligand, electron distribution between t2g and eg sets take place not in accordance with Hund’s rule. All the t2g orbitals are filled with six electrons.
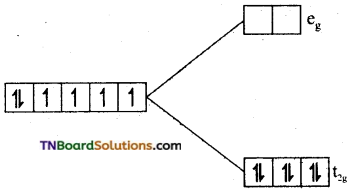
Since there is no unpaired electron, the complex is diamagnetic.
![]()
Question 64.
Give the oxidation state, ‘d’ orbital occupation and coordination number of the central metal ion in the following complexes.
(i) K3[Co(C2O4)3],
(ii) (NH4)[CoF4],
(iii) Cis – [Cr(en)2Cl2]Cl,
(iv) [Mn(H2O)6]SO4.
Answer:
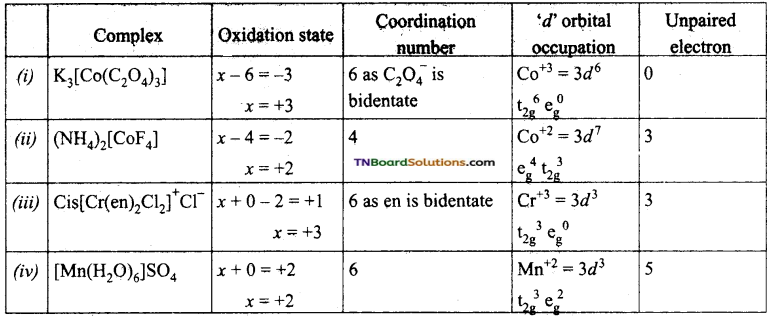
Question 65.
With the help of crystal field theory, product the number of unpaired electrons in [Fe(CN)6]-4 and [Fe(H6O)2]+2 complexes.
Answer:
In both complexes, Fe is present in +2 oxidation state and has d6 configuration. As CN– is a strong field ligand, these electrons will pair up giving the configuration t2g6 eg0. As there are no unpaired electrons, the complex is paramagnetic. But H2O is a weak ligand and electron filling follow Hund’s rule i.e., t2g4 eg2. It has 4 unpaired electron and hence paramagnetic.
Question 66.
CO is stronger ligand than NH3 for many metals. Explain giving appropriate reason.
Answer:
Ligands such as CO, CN–, NO+ have empty π orbitals which overlap with the filled d orbitals (t2g) of transition metals forming π bonds (back bonding). These π interactions increase the value of Δo. This account for the position
of these ligands as strong ligand. NH3 cannot form π bonds by back bonding.
Question 67.
What is meant by the stability of a coordination compound in solution?
Answer:
The stability of a complex in a solution refers to the degree of association between two species involved in a state of equilibrium. The magnitude of the equilibrium constant for the association is a quantitative measure of stability.
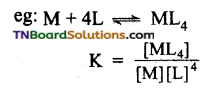
For the above equilibrium, the larger the stability constant, the higher is the proportion of ML4 that exists in the solution.
Question 68.
The stability constant of some complexes are given below:
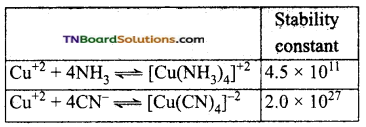
Among CN– and NH3 which is a stronger ligand? Why?
Answer:
The numerical value of the stability constant is a measure of the stability of the complex. Greater the magnitude of the stability constant more stable the complex. Thus CN– ion is a stronger ligand than ammonia.
![]()
Question 69.
The dissociation constants of [Cu(NH3)4]+2 and [CO(NH3)6]+3 are 1.10 x 10-12 and 6.2 x 10-36 respectively. Which complex would be more stable and why?
Answer:
Smaller the values of the dissociation constant more stable the complexion in solution Hence [CO(NH3)6]+3 is more stable than [Cu(NH3)4]+2 ion.
Question 70.
Calculate the overall complex dissociation equilibrium constant for the [Cu(NH3)4]+2 ion given that β4 for this complex is 2.14 x 1013.
Answer:
Overall stability constant (B4)
= 2.1 x 1013. Overall dissociation constant is the reciprocal of overall stability constant. Hence, overall dissociation constant.
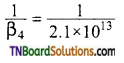
= 4.7 x 10-14.
Question 71.
Calculate the ratio of [Ag(S2O3)2]-3 and [Ag+] in 0.1 M S2O3 solution. Given the stability or formation constant of [Ag(S2O3)2]-3 is 1.0 x 1013.
Answer:
The equilibrium is
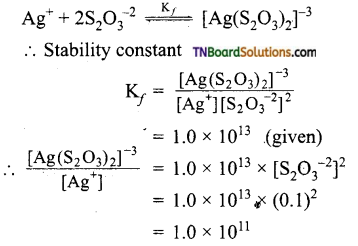
Question 72.
Mention the use of coordination compounds in metallurgy.
Answer:
- Purification of nickel by Mond’s process involves the formation of nickel carbonyl Ni(CO)4 which yields 99.5% pure nickel and decomposition.
- Coordination compounds are used in the extraction of silver and gold from their ores by forming a soluble cyano complex. These cyano complexes are reduced by zinc to yield pure metals.
Question 73.
Mention the use of complexes used as catalysts in organic reactions.
Answer:
- Wilkinson’s catalyst [(PPh3)3RhCl] is used for the hydrogenation of alkenes.
- Ziegler-Natta catalyst – [TiCl4] + Al[C2H5]3 is used in the polymerization of ethene.
- In order to get a fine and uniform deposit of superior metals (Ag, Au, Pt etc.,) over base metals, coordination complexes [Ag(CN)2]– and [Au(CN)2]– etc., are used in an electrolytic bath.
Question 74.
Mention the use of complexes in photography.
Answer:
In photography, when the developed film is washed with sodium thio sulphatesolution (hypo), the negative film gets fixed. Undecomposed AgBr forms a soluble complex called sodiumdithiosulphatoargentate(I) which can be easily removed by washing the film with water.

Question 75.
Mention the use of metal complexes in biological systems.
Answer:
- A red blood corpuscle (RBC) is composed of the heme group, which is Fe2+ – Porphyrin complex. It plays an important role in carrying oxygen from the lungs to tissues and carbon dioxide from tissues to the lungs.
- Chlorophyll, a green pigment present in green plants and algae, is a coordination complex containing Mg2+ as a central metal ion surrounded by a modified Porphyrin ligand called a corrin ring. It plays an important role in photosynthesis, by which plants converts CO2 and water into carbohydrates and oxygen.
- Vitamin B12 (cyanocobalamine) is the only vitamin consist of the metal ion. It is a coordination complex in which the central metal ion is Co+ surrounded by a Porphyrin ligand.
- Many enzymes are known to be metal complexes, they regulate biological processes. For example, Carboxypeptidase is a protease enzyme that hydrolytic enzyme important in digestion contains a zinc ion coordinated to the protein.
![]()
Question 76.
What is Cisplatin? Mention its use.
Answer:
Cisplatin is a square planar coordination complex (cis- [Pt (NH3)2C12]), in which two similar ligands are in adjacent positions. It is a platinum-based anticancer drug. This drug undergoes hydrolysis and reacts with DNA to produce various crosslinks. These crosslinks hinder DNA replication and transcription, which results in cell growth inhibition and ultimately cell death. It also crosslinks with cellular proteins and inhibits mitosis.
Question 77.
What are metal carbonyls? Mention their uses.
Answer:
Metal carbonyls are transition metal complexes of carbon monoxide, containing metal-carbonyl (M ← CO) bonds. They are used as catalysts.
Question 78.
What are mononuclear and polynuclear carbonyls? Give examples.
Answer:
Mono nuclear carbonyls contain only one metal atom.eg: Ni(CO)4, ie(CO)5, Cr(CO)6. Polynuclear carbonyls contain two or more metal atoms. They may be homonuclear or heteronuclear.
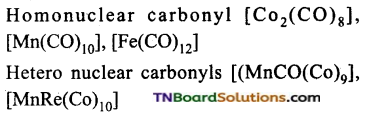
Question 79.
What are non-bridged metal carbonyls? Give examples.
Answer:

- Non bridged metal carbonyls contain only terminal carbonyls, eg: [Ni (Co)4] [Fe(Co)5] and [Cr(Co)6]
- Non bridged metal carbonyl which contains terminal carbonyls as well as metal- metal bonds, eg: Mn2(Co)10. It contains Mn—Mn bonds and also Mn ← 10 bonds.
Question 80.
What are bridged carbonyls? Give example.
Answer:
These metal carbonyls contain one or more bridging carbonyl ligands along with terminal carbonyl ligands and one or more Metal-Metal bonds. For example,
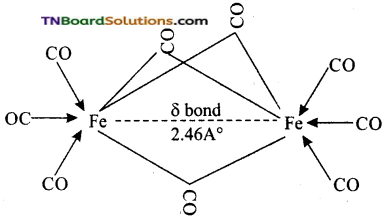
The structure of Fe2(CO)9, di-ironennea carbonyl molecule consists of three bridging CO ligands, six terminal CO groups and a single Fe-Fe bond formed by the weak coupling of the unpaired electrons present in two 3d orbitals of two Fe atoms. This bond is represented by a dotted line and is called a fractional single bond.
Choose the correct answer.
1. Which of the following complexes formed by Cu+2 ions is the most stable?

Answer:
(b)
Hint: Greater the log k value, more stable the complex.
![]()
2. When 1 mol of CrCl3 6H2O is treated with excess of AgNO3 3 mol of AgCl are obtained. The formula of the complex is:

Answer:
(d)
3. The correct IUPAC name of [Pt (NH3)2Cl2] is:
(a) Diammine dichloridoplatinum (II)
(b) Diammine dichloridoplatinum (IV)
(c) Diammine dichlorido platinum (0)
(d) Dichlorodiammine platinum (IV)
Answer:
(a)
4. The stabilisation of coordination compounds due to chelation is called chelate effect. Which of the following is the most stable complex species?

Answer:
(c)
Hint: C2O4-2 is a bidentate ligand forming chelate.
5. The compounds [Co(SO4)(NH3)5] Br and [Co(SO4)(NH3)5]Cl represent:
(a) linkage isomerism
(b) ionisation isomerism
(c) coordination isomerism
(d) no isomerism
Answer:
(d)
6. Which of the following species is not expected to be a ligand?
(a) NO
(b) NH4+
(c) NH2CH2CH2NH2
(d) Co
Answer:
(b)
Hint: It has no lone pair of electrons.
![]()
7. The oxidation state of Fe in the brown ring complex [Fe(H2O)5NO]SO4 is:
(a) +1
(b) +2
(c) +3
(d) +4
Answer:
(a)
Hint: [Fe(H2O)5NO]+2 : x + 0 + 1 = 2
x = 1.
8. [CO(NH3)4 (NO2)2]Cl exhibits:
(a) ionisation isomerism, geometrical isomerism and optical isomerism.
(b) linkage isomerism, geometrical isomerism and optical isomerism.
(c) linkage isomerism, ionisation isomerism and optical isomerism.
(d) linkage isomerism, ionisation isomerism and geometrical isomerism.
Answer:
(d)
Hint:
9. Which of the following complex ions has geometrical isomers?
Answer:
(c)
Hint: Geometrical isomerism is shown by disubstituted octahedral complexes such as [Co(NH3)2 (en)2]+3
10. The correct statement with respect to the complexes Ni(CO)4 and [Ni(CN)4]-2 is:
(a) nickel is in the same oxidation state in both.
(b) both have tetrahedral geometry
(c) both have square planar geometry
(d) have tetrahedral and square planar geometry respectively.
Answer:
(d)
Hint: Ni(CO)4: sp3hybridisation: Tetrahedral
[Ni(CN)4]-2: dsp2 hybridisation: square planar.
11. When 0.01 mole of a cobalt complex is treated with excess AgNO3 solution, 4.305g of silver chloride is precipitated. The formula of the complex is:
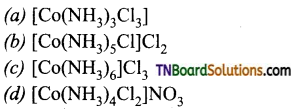
Answer:
(c)
Hint: 4.305g AgCl = \(\frac{4.305}{143.5}\) mol = 0.03 mol As 0.01 mol of the complex gives 0.03 mol of AgCl this shows that there are 3 ionisable chlorine atoms i.e., c.
![]()
12. Amongst Ni(CO)4, [Ni(CN)4]-2 and [NiCl4]-2
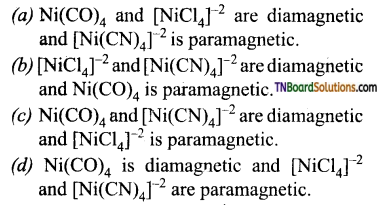
Answer:
(c)
Hint: Ni(CO)4 and [Ni(CN)4]-2 do not contain any unpaired electrons while [NiCl4]-2 contain two unpaired electrons.
13. Which of the following complexes are not correctly matched with the hybridisation of their central metal ion?
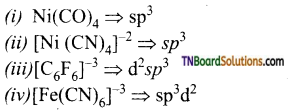
(a) (i) and (ii)
(b) (ii), (iii) and (iv)
(c) (i), (ii) and (iii)
(d) (ii) and (iv)
Answer:
(b)
Hint:
is a weak ligand and cannot force ‘d’ electrons to pair up forming outer orbital complex.
14. Which of the following complexes has a minimum magnitude of ΔO?

Answer:
(c)
Hint:
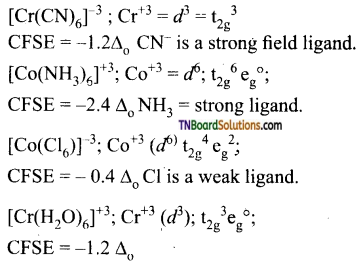
Hence, the minimum CFSE is for [CoCl6]-3.
15. Hybridisation, shape and magnetic moment of K3 [Co(CO3)3] is:
(a) d2sp3, octahedral, 4.9 BM
(b) sp3d2, octahedral, 4.9 BM
(c) dsp2, square planar, 4.9 BM
(d) sp3, tetrahedral, 4.9 BM
Answer:
(b)
Hint: In each case, the magnetic moment is 4.9 BM, which shows that each of them has 4 unpaired electrons. Further, in the given complex, oxidation state of cobalt is +3. Hence, it will have d6 configuration.

Thus the possible hybridisation in sp3d2. i.e., outer orbital (high spin) octahedral complex.
![]()
16. The spin only magnetic moment value (in Bohr magneton) of Cr(CO)6 is:
(a) 0
(b) 2.84
(c) 4.90
(d) 5.92
Answer:
(d)
Hint.In Cr(CO)6, Cr is in zero oxidation state, [Ar]3d5 4s1. As CO is a strong ligand, all the six electrons will pair up i.e, there will be no unpaired electron. Hence, u = 0.
17. Among the following complexes (K – P)
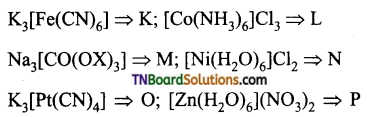
the diamagnetic complexes are:
(a) K, L, M, N
(b) K, M, O, P
(c) L, M, O, P
(d) L, M, N, O
Answer: (c)
Hint:
K = K3[Fe(CN)6] : Fe+3 (3d5) d2sp3 hybridisation.
No. of unpaired electron = 1; Paramagnetic
L = [CO(NH3)6Cl3]; CO+3 (3d6): d2sp3 hybridisation.
No. of unpaired electrons = 0; Diamagnetic
M: Na2 [CO(oX)3]: CO+3 (3d6): d2sp3 hybridisation.
No. of unpaired electron = 0; Diamagnetic
N: [Ni(H2O)6] Cl2 ; Ni+3 (3d8): sp3d2 hybridisation.
No. of unpaired electron = 2; paramagnetic
O: K2 [Pt (CN)1]; Pt+2 (3d8): dsp2 hybridisation.
No. of unpaired electron = 0; Diamagnetic.
P: [Zn(H2O)6] (NO3)2; Zn+2 (3d10); sp3d2 hybridisation;
No. of unpaired electron = 0; Diamagnetic.
Hence, diamagnetic complexes are L, M, O and P.
18. Which of the following facts about the complex [Cr(NH3)6] Cl3 is wrong?
(a) The complex is paramagnetic
(b) The complex is an outer orbital complex
(c) The complex gives white precipitate with silver nitrate solution.
(d) The complex involves d2sp3 hybridisation and is octahedral in shape.
Answer:
(b)
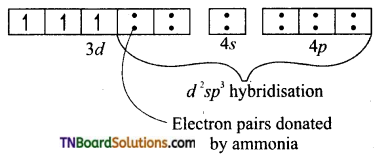
Hint: In [Cr(NH3)6]Cl3, Cr+3(3d3): d2sp3 hybridisation. No. of unpaired electron = 3 paramagnetic. The complex is inner orbital complex as it involves (n -1) d orbitals for hybridisation. [Cr(NH3)6]+3
19. The ‘d’ electron configuration of Cr+2, Mn+2, Fe+2 and Co+2 are d4, d5, d6 and d1 respectively. Which one of the following will exhibit minimum paramagnetic behaviour?

Answer:
(d)
Hint:
In [Mn(H2O)6]+2, Mn+2 = 3d5
So, the number of unpaired electron = 5.
In [C(H2O)6]+2, Fe+2 = 3d6
So, the number of unpaired electron = 4.
In [Co(H2O)6]+2, Co+2 = 3d7
So, the number of unpaired electron = 3.
In [Cr(H2O)6]+2, Cr+2 = 3d4
So, the number of unpaired electron = 4.
All these show sp3d2 hybridisation forming outer orbital complexes. Hence, minimum paramagnetic behaviour is shown by [Co(H2O)6]+2
![]()
20. Crystal field stabilisation energy for high spin d4 octahedral complex is:
(a) -0.6 Δo
(b) -1.8 Δo
(c) -1.66 Δo + P
(d) -1.2 Δo
Answer:
(a)
Hint: d4 octahedral complex has the configuration t2g3 eg1
CFSE = (-0.4x + 0.6y) Δo
x = no. of electron in t2g
y = no. of electron in eg
CFSE = [-0.4 x 3+ 0.6 x 1] Δo
= [-1.2 + 0.6] Δo
= – 0.6 Δo
21. In spectrochemical series, chlorine is above H2O i.e, Cl > H2O. This is due to:
(a) Good π acceptor properties of chlorine
(b) Strong σ donor and good π acceptor properties of chlorine.
(c) Good π donor properties of chlorine.
(d) Larger size of chlorine than H2O.
Answer:
(c)
22. The magnitude of crystal field stabilisation energy (CFSE or Δt) in tetrahedral complexes is considerably less than in the octahedral field. Because
(a) There are only 4 ligands instead of six.
So the ligand field in only 2/3 the size, hence Δt, is only 2/3 the size.
(b) the direction of orbitals does not coincide with the direction of the ligands. This reduces the crystal field stabilisation energy (Δt) by further 2/3.
(c) both (a) and (b) are correct
(d) both (a) and (b) are wrong.
Answer:
(c)
23. Which of the following complex ions is expected to absorb visible light?
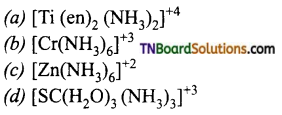
(At. No: Zn = 30, Sc = 21, Ti = 22; Cr = 24) ,
Answer:
(b)
Hint: d0 (Sc+3, Ti+4) and d10 (Zn+2) species will not absorb visible light and are colurless whereas Cr+3 (d3) can undergo d-d transition on absorption of visible light of particular wave length and will be coloured.
![]()
24. Which of the following is high spin complex?
(a) [CoCl6]-3
(b) [FeF6]-3
(c) [Co(NH3)6]+2
(d) All of these.
Answer:
(d)
Hint: All the given ligands are weak field ligands. Hence they form high spin complexes.
25. Which one of the following ligand is capable of forming a low spin as well as high spin complex?
(a) CO
(b) F
(c) NH3
(d) CN–
Answer:
(c)
Hint:CO and CN– are strong ligands and F– is a weak ligand. NH3 is neither very weak nor very strong ligand.
26. Complexes with halide ions are generally:
(a) low spin complexes
(b) high spin complexes
(c) both (a) and (b)
(d) neither (a) and (b)
Answer:
(b)
Hint: Halide ions have low CFSE (A) values. They cannot pair up the electrons of the metal atom / ion. Hence they form high spin complexes.
27. Which of the following shall form an octahedral complex?
(a) d4 (low spin)
(b) d8 (high spin)
(c) d6 (low spin)
(d) all of these
Answer:
(c)
Hint: In d6 (low spin) electrons pair up thereby making two empty ‘d orbitals of the metal atom / ion and undergo d2sp3 hybridisation.
28. Match the entities of column I with appropriate entities of column II.
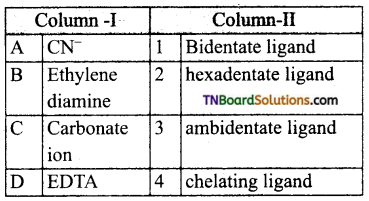
Code:
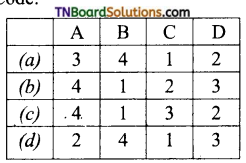
Answer:
(a)
![]()
29. Assertion (A): The complex [CO(NH3)3Cl3] does not give precipitate with AgNO3.
Reason (R): The complex does not contain counter ions.
(a) If both assertion and reason are true and reason is the correej explanation of assertion.
(b) If both assertion and reason are true, but reason is not the correct explanation of assertion.
(c) If assertion is true but reason is false.
(d) If both assertion and reason are false.
Answer:
(a)
30. Assertion (A): The [Ni(en)3]Cl2 (en = ethylene diamine) has lower stability than (Ni (NH3)6] Cl2.
Reason (R): In [Ni(en)3] Cl2, the geometry of nickel is trigonal bi-pyramidal.
(a) If both assertion and reason are true and the reason is the correct explanation of assertion.
(b) If both assertion and reason are true, but the reason is not the correct explanation of assertion.
(c) If the assertion is true but the reason is false.
(d) If both assertion and reason are false.
Answer:
(d)
Hint:
Correct assertion: [Ni(en)3]Cl2 has greater Stability than [Ni (NH3)6]Cl2.
Correct Reason: [Ni (en)3]Cl2 has a bidentate ligand (en). It forms chelates.