Tamilnadu State Board New Syllabus Samacheer Kalvi 11th Chemistry Guide Pdf Chapter 10 Chemical Bonding Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 11th Chemistry Solutions Chapter 10 Chemical Bonding
11th Chemistry Guide Chemical Bonding Text Book Back Questions and Answers
Textbook Evaluation:
I. Choose the best answer:
Question 1.
In which of the following compound does the central atom obey the octet rule?
a) XeF4
b) AlCl3
c) SF6
d) SCl2
Answer:
d) SCl2
Question 2.
In the molecule OA = C = OB, the formal charge on OA, C and OB are respectively.
a) -1, 0, +1
b) +1, 0, -1
c) -2, 0, +2
d) 0, 0, 0
Answer:
d) 0, 0, 0
Question 3.
Which of the following is electron deficient?
a) PH3
b) (CH3)2
c) BH3
d) NH3
Answer:
c) BH3
Question 4.
Which of the following molecule contain no π bond?
a) SO2
b) NO2
c) CO2
d) H2O
Answer:
d) H2O
Question 5.
The ratio of number of sigma (σ) bond and pi (π) bonds in 2 – butynal is
a) 8/3
b) 5/3
c) 8/2
d) 9/2
Answer:
a) 8/3
![]()
Question 6.
Which one of the following is the likely bond angles of sulphur tetrafluo-ride molecule?
a) 120°, 80°
b) 109°28’
c) 90°
d) 89°, 117°
Answer:
d) 89°, 117°
Question 7.
Assertion:
Oxygen molecule is paramagnetic.
Reason :
It has two unpaired electron in its bonding molecular orbital
a) both assertion and reason are true and reason is the correct explanation of assertion.
b) both assertion and reason are true but reason is not the correct explanation of assertion.
c) assertion is true but reason is false.
d) both assertion and reason are false.
Answer:
c) assertion is true but reason is false.
Question 8.
According to Valence bond theory, a bond between two atoms is formed when
a) fully filled atomic orbitals overlap
b) half filled atomic orbitals overlap
c) non – bonding atomic orbitals overlap
d) empty atomic orbitals overlap
Answer:
b) half filled atomic orbitals overlap
Question 9.
In ClF3, NF3 and BF3 molecules the chlorine, nitrogen and boron atoms are
a) sp3 hybridised
b) sp3, sp3 and sp2 respectively
c) sp3 hybridised
d) sp3d, sp3 and sp hybridised respectively
Answer:
d) sp3d, sp3 and sp hybridised respectively
Question 10.
When one s and three p orbitals hybridise,
a) four equivalent orbitals at 90° to each other will be formed
b) four equivalent orbitals at 109°28’ to each other will be formed
c) four equivalent orbitals, that are lying the same plane will be formed
d) none of these
Answer:
b) four equivalent orbitals at 109°28’ to each other will be formed
![]()
Question 11.
Which of these represents the correct order of their increasing bond order.
a) C2+ < C22- < O22- < O2
b) C22- < C2+ < O2 < O22-
c) O22- < O2 < C22- < C2+
d) O22- < C2+ < O2 < C22-
Answer:
d) O22- < C2+ < O2 < C22-
Question 12.
Hybridisation of central atom in PCl5 involves the mixing of orbitals.
a) s, Px, Py, dx2, dx2 – y2
b) s, px, py, pxy, dx2 – y2
c) s, px, py, pz, dx2 – y2
d) s, px, Py, dxy, dx2 – y2
Answer:
c) s, px, py, pz, dx2 – y2
Question 13.
The correct order of O – O bond length in hydrogen peroxide, ozone and oxygen is
a) H2O2 > O3 > O2
b) O2 > O3 > H2O2
c) O2 > H2O2 > O3
d) O3 > O2 > H2O2
Answer:
b) O2 > O3 > H2O2
Question 14.
Which one of the following is diamagnetic?
a) O2
b) O22-
c) O2+
d) None of these
Answer:
b) O22-
Question 15.
Bond order of a species is 2.5 and the number of electrons in its bonding molecular orbital is formed to be 8. The no. of electrons in its antibonding molecular orbital is
a) three
b) four
c) Zero
d) can not be calculated from the given information
Answer:
a) three
![]()
Question 16.
Shape and hybridisation of IF5 are
a) Trigonal bipyramidal, sp3d2
b) Trigonal bipyramidal, sp3d
c) Square pyramidal, sp3d2
d) Octahedral, sp3d2
Answer:
c) Square pyramidal, sp3d2
Question 17.
Pick out the incorrect statement from the following:
a) sp3 hybrid orbitals are equivalent and are at an angle of 109°28’ with each other
b) dsp2 hybrid orbitals are equivalent and bond angle between any two of them is 90°
c) All five sp3d hybrid orbitals are not equivalent out of these five sp3d hybrid orbitals, three are at an angle of 120° remaining two are perpendicular to the plane containing the other three
d) none of these
Answer:
c) All five sp3d hybrid orbitals are not equivalent out of these five sp3d hybrid orbitals, three are at an angle of 120° remaining two are perpendicular to the plane containing the other three
Question 18.
The molecules having same hybridisation, shape and number of lone pairs of electrons are
a) SeF4, XeO2F2
b) SF4, XeF2
c) XeOF4, TeF4
d) SeCl4, XeF4
Answer:
a) SeF4, XeO2F2
Question 19.
In which of the following molecules / ions BF3, NO2– H20 the central atom is sp2 hybridised?
a) NH2– and H2O
b) NO2– and H2O
c) BF3 and NO2–
d) BF3 and NH2–
Answer:
c) BF3 and NO2–
Question 20.
Some of the following properties of two species, NO3– and H3O+ are described below. Which one of them is correct?
a) dissimilar in hybridisation for the central atom with different structure
b) isostructural with same hybridisation for the central atom.
c) different hybridisation for the central atom with same structure.
d) none of these
Answer:
a) dissimilar in hybridisation for the central atom with different structure
![]()
Question 21.
The types of hybridisation on the five-carbon atom from right to left in the, 2,3 pentadiene.
a) sp3, sp2, sp, sp2, sp3
b) sp3, sp, sp, sp, sp3
c) sp2, sp, sp2, sp2, sp3
d) sp3, sp3, sp2, sp3, sp3
Answer:
a) sp3, sp2, sp, sp2, sp3
Question 22.
XeF2 is isostructural with
a) SbCl2
b) BaCl2
c) TeF2
d) ICl2–
Answer:
d) ICl2–
Question 23.
The percentage of s-character of the hybrid orbitals in methane, ethane, ethene, and ethyne are respectively
a) 25, 25, 33.3, 50
b) 50, 50, 33.3, 25
c) 50, 25, 33.3, 50
d) 50, 25, 25, 50
Answer:
a) 25, 25, 33.3, 50
Question 24.
Of the following molecules, which have shape similar to carbondioxide?
a) SnCl2
b) NO2
c) C2H2
d) All of these
Answer:
c) C2H2
Question 25.
According to VSEPR theory, the repulsion between different parts of electrons obey the order
a) 1. p – 1. p > b. p – b. p > 1. p – b. p
b) b. p – b. p > b. p – 1. p > 1. p – b. p
c) 1. p – 1. p > b. p – 1. p > b. p – b. p
d) b. p – b. p > 1. p – 1. p > b. p – 1. p
Answer:
c) 1. p – 1. p > b. p – 1. p > b. p – b. p
![]()
Question 26.
Shape of ClF3 is
a) Planar triangular
b) Pyramidal
c) “T” Shaped
d) none of these
Answer:
c) “T” Shaped
Question 27.
Non – Zero dipole moment is shown by
a) CO2
b) p – dichlorobenzene
c) carbontetrachloride
d) water
Answer:
d) water
Question 28.
Which of the following conditions is not correct for resonating structures?
a) the contributing structure must have the same number of unpaired electrons
b) the contributing structures should have similar energies
c) the resonance hybrid should have higher energy than any of the contributing structure.
d) none of these
Answer:
c) the resonance hybrid should have higher energy than any of the contributing structure.
Question 29.
Among the following, the compound that contains, ionic, covalent, and Coordinate linkage is
a) NH4Cl
b) NH3
c) NaCl
d) none of these
Answer:
a) NH4Cl
Question 30.
CaO and NaCl have the same crystal structure and approximately the same radii. If U is the lattice energy of NaCl, the approximate lattice energy of CaO is
a) U
b) 2U
c) U /2
d) 4U
Answer:
d) 4U
![]()
II. Write brief answer to the following questions:
Question 31.
Define the following:
i) Bond order
ii) Hybridisation
iii) σ – bond
Answer:
i) Bond order:
The number of bonds formed between the two bonded atoms in a molecule is called the bond order.
ii) Hybridisation:
Hybridisation is the process of mixing of atomic orbitals of the same atom with comparable energy to form an equal number of new equivalent orbitals with the same energy.
iii) σ – bond:
When two atomic orbitals overlap linearly along the axis, the resultant bond is called a sigma (σ) bond.
Question 32.
What is a pi – bond?
Answer:
When two atomic orbitals overlaps sideways, the resultant covalent bond is called a pi (π) bond. When we consider x – axis as molecular axis, the py – py and pz – pz overlaps will result in the formation of a π – bond.
Question 33.
In CH4, NH3 and H2O, the central atom undergoes sp3 hybridization – yet their bond angles are different. Why?
Answer:
According to VSEPR theory, as H2O has two lone pairs so it repels the bond pairs much more and makes bond angle shorter of 104.5 degrees, and as NH3 has one lone pair that repels the three bond pairs but not much effectively and strongly as two lone pairs of water repel one bond pair.
So the bond angle between Hydrogen atom of ammonia is 107.5 greater than that of water. Similarly, methane molecule have no lone pair and bond pair repels each other with the equal bond angle between two adjacent hydrogen atoms becomes 109°.28′.
Question 34.
Explain Sp2 hybridization in BF3.
Answer:
Consider boron trifluoride molecule. The valence shell electronic configuration of boron atom is [He]2s2 2p1.
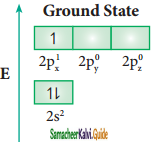
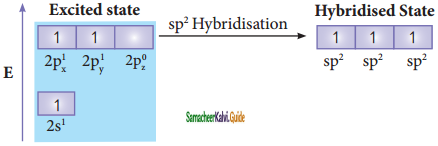
In the ground state boron has only one unpaired electron in the valence shell. In order to form three covalent bonds with fluorine atoms, three unpaired electrons are required. To achieve this, one of the paired electrons in the 2s orbital is promoted to the 2py orbital in the excite state. In boron, the s orbital and two p orbitals (px and py) in the valence shell hybridises, to generate three equivalent sp2 orbitals as shown in the Figure. These three orbitals lie in the same xy plane and the angle between any two orbitals is equal to 120°.
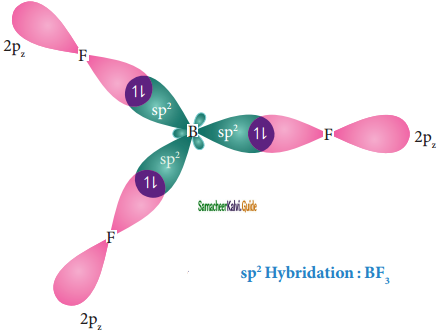
Overlap with 2pz orbitals of flourine:
The three sp2 hybridised orbitals of boron now overlap with the 2pz orbitals of fluorine (3 atoms). This overlap takes place along the axis as shown below.
![]()
Question 35.
Draw the M.O diagram for oxygen molecule calculate its bond order and show that O2 is paramagnetic.
Answer:
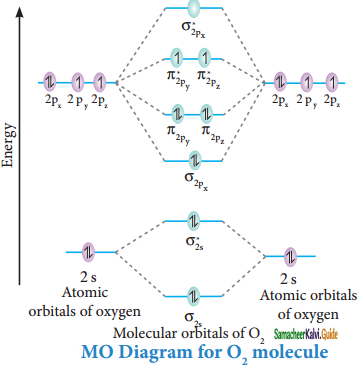
Electronic configuration of O atom:
1s2 2s2 2p4
Electronic configuration of O2 molecule:
σ1s2, σ1s*2, σ2s2, σ2s*2, σ2px2, π2py2 π2pz2 π2py*1 π2pz*1
Bond order = \(\frac{N_{b}-N_{a}}{2}\)
= \(\frac{10-6}{2}\) = 2
Molecule has two unpaired electrons hence it is paramagnetic.
Question 36.
Draw MO diagram of CO and calculate its bond order.
Answer:
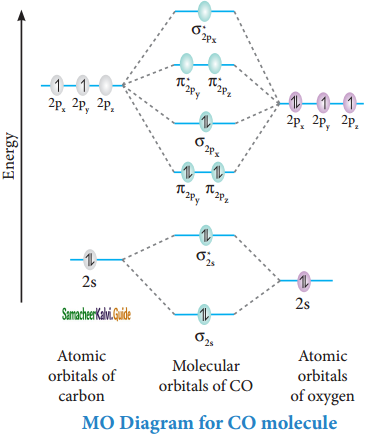
Bonding in some hetero nuclear di-atomic molecules:
Molecular orbital diagram of Carbon monoxide molecule (CO)
Electronic configuration of C atom: 1s2 2s2 2p2
Electronic configuration of O atom: 1s2 2s2 2p4
Electronic configuration of CO molecule :
σ1s2, σ1s*2, σ2s2, σ2s*2, π2py2, π2pz2 σ2px2
Bond order = \(\frac{N_{b}-N_{a}}{2}\)
= \(\frac{10-4}{2}\)
= 3
Molecule has no unpaired electrons hence it is diamagnetic.
Question 37.
What do you understand by Linear combination of atomic orbitals in MO theory?
Answer:
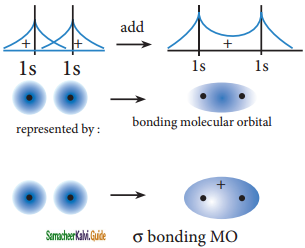
The wave functions for the molecular orbitals can be obtained by solving Schrodinger wave equation for the molecule. Since solving the Schrodinger equation is too complex, approximation methods are used to obtain the wave function for molecular orbitals. The most common method is the linear combination of atomic orbitals (LCAO).
We know that the atomic orbitals are represented by the wave function ψ. Let us consider two atomic orbitals represented by the wave function ψA and ψB with comparable energy, combines to form two molecular orbitals. One is bonding molecular orbital(ψbonding) and the other is antibonding molecular orbital (ψantibonding) The wave functions for these two molecular orbitals can be obtained by the linear combination of the atomic orbitals ψA and ψB as below,
ψbonding = ψA + ψB;
ψantibonding = ψA – ψB
The formation of bonding molecular orbital can be considered as the result of constructive interference of the atomic orbitals and the formation of anti-bonding molecular orbital can be the result of the destructive interference of the atomic orbitals. The formation of the two molecular orbitals from two is orbitals is shown below.
Constructive interaction:
The two 1s orbitals are in phase and have the same sign,
Destructive interaction:
The two orbitals are out phase
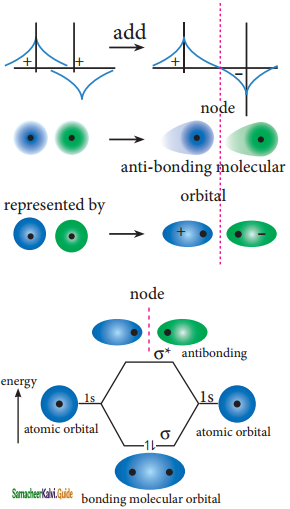
![]()
Question 38.
Discuss the formation of N2 molecule using MO theory.
Answer:
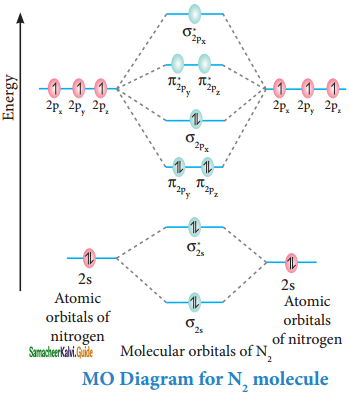
Molecular orbital diagram of nitrogen molecule (N2)
Electronic configuration of N atom 1s2 2s2 2p3
Electronic configuration of N2 molecule:
σ1s2, σ1s*2, σ2s2, σ2s*2, π2py2, π2pz2 σ2px2
Bondorder = \(\frac{N_{b}-N_{a}}{2}\)
= \(\frac{10-4}{2}\) = 3
Molecule has no unpaired electrons hence it is diamagnetic.
Question 39.
What is dipole moment?
Answer:
The polarity of a covalent bond can be measured in terms of dipole moment which is defined as
μ = q × 2d
Where μ is the dipole moment, q is the charge and 2d is the distance between the two charges. The dipole moment is a vector and the direction of the dipole moment vector points from the negative charge to positive charge.
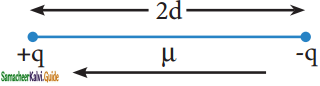
representation of dipole
The unit of dipole moment is coloumb meter (C m). It is usually expressed in Debye unit (d). The conversion factor is 1 Debye = 3.336 × 10-30 C m.
Question 40.
Linear form of carbondioxide molecule has two polar bonds. Yet the molecule has Zero dipole moment. Why?
Answer:
The linear form of carbon dioxide has zero dipole moment, even though it has two polar bonds. In CO2, the dipole moments of two polar bonds (CO) are equal in magnitude but have opposite direction. Hence, the net dipole moment of the CO2 is,
μ = μ1 + μ2 = μ1 + (-μ1) = 0
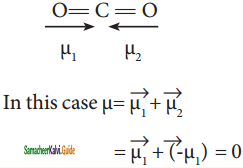
Question 41.
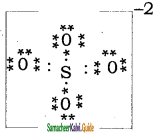
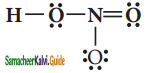
Draw the Lewis structures for the following species.
(i) NO3–
(ii) SO42-
(iii) HNO3
(iv) O3
Answer:
(i) NO3–

(ii) SO42-
(iii) HNO3
(iv) O3

![]()
Question 42.
Explain the bond formation if BeCl2 and MgCl2.
Answer:
Bond formation of BeCl2:
Be = 4;
Electronic configuration of Be atom is = 1s2 2s2
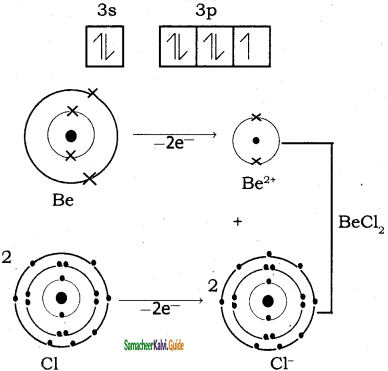
Bond formation of MgCl2:
Mg (Z = 12), 1s2 2s2 2p6 3s2 it loses two of its valence electron and became Mg2+ with inert gas configuration Neon.
The chlorine accept one electron in its valence shell and because Cl– ion with Ar electron configuration.
Mg + Cl2 → Mg2+ + 2 Cl– → MgCl2
Magnesium cation and two chlorides are attracted by strong electro static force to form MgCl2 crystals.
Mg = 12,
Electronic configuration: 1s2 2s2 2p6 3s2
Mg+2:
Electronic configuration: 1s2 2s2 2p6 3s0
Cl = 17, 1s2 2s2 2p6 3s2 3p5
Cl–:
Electronic configuration: 1s2 2s2 2p6 3s2 3p6
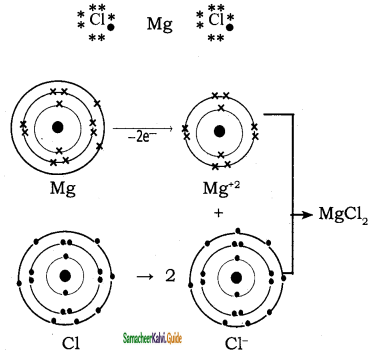
Question 43.
Which bond is stronger σ or π? Why?
Answer:
σ bond is stronger than π bond. A sigma bond is formed by head on overlapping of orbital is more effective. Hence it is stronger bond. But pi bonds are formed by sidewise overlapping of orbitals. The sidewise overlapping of orbitals is less effective than head on overlapping. Hence it is a weaker bond.
Question 44.
Define bond energy.
Answer:
The bond enthalpy is defined as the minimum amount of energy required to break one mole of a particular bond in molecules in their gaseous state. The unit of bond enthalpy is kJ mol-1.
Question 45.
Hydrogen gas is diatomic where as inert gases are monoatomic – Explain on the basis of MO theory.
Answer:
The molecular orbital electronic configuration of hydrogen molecule is (σ1s2). The molecular orbital energy level diagram of H2 molecule is given in
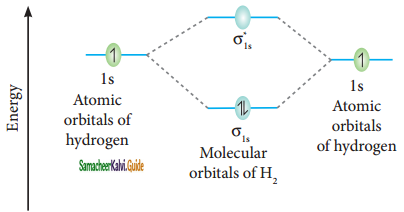
Here, N2 = 2, Na = 0
Bond order = \(\frac{N_{b}-N_{a}}{2}=\frac{2-0}{2}\) = 1
He2: σ1s2 σ1s*2
The molecular orbital energy level diagram of He2 (hypothetical) is given in
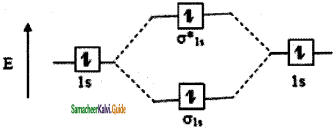
Here, Nb = 2 and Na = 2
Bond order = \(\frac{N_{b}-N_{a}}{2}=\frac{2-2}{2}\) = 0
As the bond order for He2 comes out to formed between two hydrogen atoms. But as the bond order of helium is zero, there is no bond between helium atoms and hence it is mono atomic.
Result:
As the bond order of H2 molecule is one, it is diatomic and a single bond is formed between two hydrogen atoms. But as the bond order of helium is zero, there is no bond between helium atoms and hence it is mono atomic.
![]()
Question 46.
What is the Polar Covalent bond? Explain with example.
Answer:
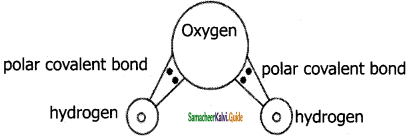
Polar covalent bond is a chemical bond in which the electrons required to form a bond is unequally shared between two atoms. The atom which is more electronegative attracts more electrons from the bonded pair than the other atom. As a result there is a slight separation of charges in a molecule in which more electronegative atom (comparatively) carries a slight negative charge and less electronegative atom carries a positive charge. The bonds formed between two atoms have a permanent electric dipole.
In water (H2O) molecule, covalent bond exists between Hydrogen and Oxygen. Being more electronegative Oxygen carries negative charge and Hydrogen carries positive charge. In a water molecule Oxygen carries negative charge (anionic in nature) and hydrogen’s carries positive charge (cationic in nature) which forms a polar covalent bond. The size of an oxygen atom is comparatively higher than a hydrogen atom, hence it polarizes the molecule towards itself i.e., it attracts shared pair of bonding electrons towards itself and makes the bond to be more polarized. Hydrogen which is comparatively a small sized cation makes the bond to be polarized better.
Question 47.
considering x-axis as molecular axis which out of the following will form a sigma bond.
i) 1s and 2py
ii) 2px and 2py
iii) 2px and 2pz
iv) 1s and 2pz
Answer:
i) 1s and 2py: No sigma bond
ii) 2px and 2py: sigma bond
iii) 2px and 2pz: No sigma bond
iv) 1s and 2pz: No sigma bond
Question 48.
Explain resonance with reference to carbonate ion.
Answer:
It is evident from the experimental results that all carbon – oxygen bonds in carbonate ions are equivalent. The actual structure of the molecules is said to be resonance hybrid, an average of these three resonance forms. It is important to note that carbonate ion does not change from one structure to another and vice versa. is not possible to picturise the reasonance hybrid by drawing a single Lewis structure. However, the following structure gives a qualitative idea about the correct structure.
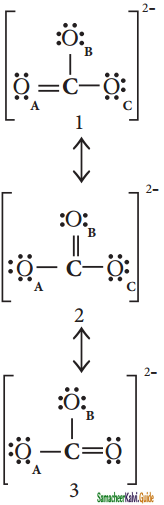
(b) Resonance structure of CO32-:
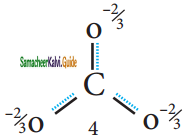
Resonance Hybrid structure of CO32-:
It is found that the energy of the resonance hybrid (structure 4) is lower than that of all possible canonical structures (Structure 1, 2 & 3). The difference in energy between structure 1 or 2 or 3, (most stable canonical structure) and structure 4 (resonance hybrid) is called resonance energy.
Question 49.
Explain the bond formation in ethylene and acetylene.
Answer:
Bonding in ethylene:
The bonding in ethylene can be explained using hybridization concept. The molecular formula of ethylene is C2H4. The valency of carbon is 4. The electronic configuration of valence shell of carbon in ground state is [He] 2s2 2px1 2py1 2pz0. To satisfy the valency of carbon promote an electron from 2s orbital to 2pz orbital in the excited state.
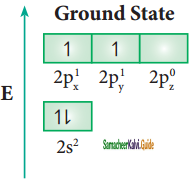

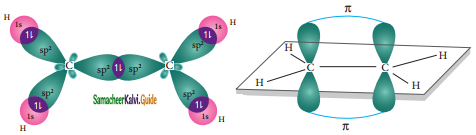
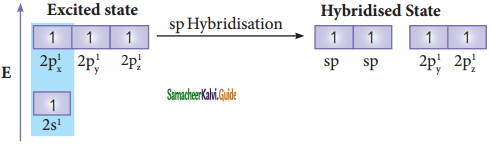
In ethylene both the carbon atoms undergoes sp2 hybridisation involving 2s, 2px, and 2py, orbitals, resulting in three equivalent sp2 hybridised orbitals lying in the xy plane at an angle of 120° to each other. The unhybridised 2pz orbital lies perpendicular to the xy plane.
Formation of sigma bond:
One of the sp2 hybridised orbitals of each carbon lying on the molecular axis (x-axis) linearly overlaps with each other resulting in the formation a C-C sigma bond. Other two sp2 hybridised orbitals of both carbons linearly overlap with the four is orbitals of four hydrogen atoms leading to the formation of two C-H sigma bonds on each carbon.
Formation of sigma bond:
The unhybridized 2pz orbital of both carbon atoms can overlap only sideways as they are not in the molecular axis. This lateral overlap results in the formation of a pi bond between the two carbon atoms as shown in the figure.
Bonding in acetylene :
Similar to ethylene, the bonding in acetylene can also be explained using hybridisation concept. The molecular formula of acetylene is C2H2. The electronic configuration of valence shell of carbon in ground state is [He] 2s2 2px1 2py1 2pz0. To satisfy the valency of carbon promote an electron from 2s orbital to 2pz orbital in the excited state.
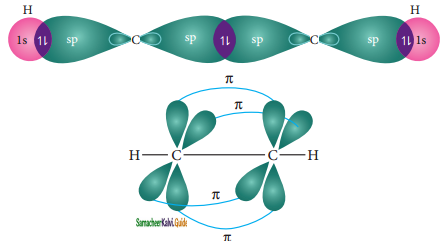
In acetylene molecule, both the carbon atoms are in sp hybridized state. The 2s and 2px orbitals, resulting in two equivalent sp hybridized orbitals lying in a straight line along the molecular axis (x-axis). The unhybridized 2py and 2pz orbitals lie perpendicular to the molecular axis.
Formation of sigma bond:
One of the two sp hybridized orbitals of each carbon lineraly overlaps with each other resulting in the formation a C – C sigma bond. The other sp hybridized orbitals of both carbons linearly overlap with the two 1s orbitals of two hydrogen atoms leading to the formation of one C – H sigma bonds on each carbon.
Formation of pi bond :
The unhybridized 2py and 2pz orbitals of each carbon overlap sideways. This lateral overlap results in the formation of two pi bonds (py – py and pz – pz) between the two carbon atoms as shown in the figure.
Question 50.
What type of hybridisations are possible in the following geometries?
a) octahedral b) tetrahedral c) square planar
Answer:
a) octahedral: sp3d2
b) tetrahedral: sp3
c) square planar: dsp2
![]()
Question 51.
Explain VSEPR theory. Applying this theory to predict the shapes of IF7 and SF6.
Answer:
Lewis concept of structure of molecules deals with the relative position of atoms in the molecules and sharing of electron pairs between them. However, we cannot predict the shape of the molecule using Lewis concept. Lewis theory in combination with VSEPR theory will be useful in predicting the shape of molecules.
IF7:
Iodine has 7 valence electrons in the valence shell. In excited state it has 6 valency electrons by 1st and 2nd excited state & under sp3d2, hybridization and combines with 7 Fluorine to get pentagonal bipyramidal shape. There are no lone pairs.
I = ns2 np5 nd0
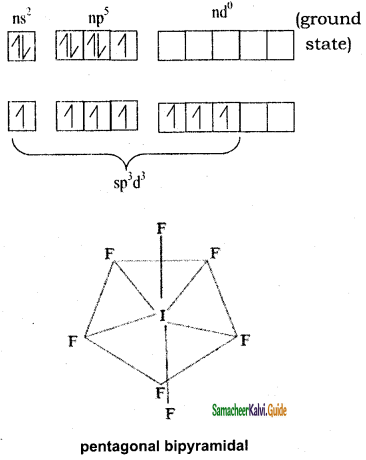
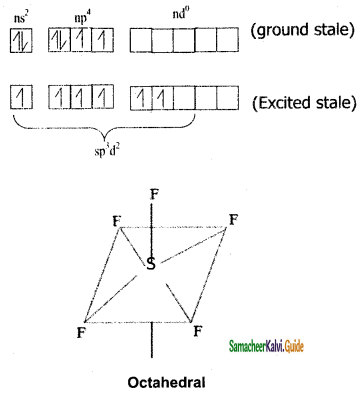
SF6:
Sulphar attain six valence electron in 1st and 2nd excited states undergoes sp3d2 hybridization and combines with six ‘F’ atoms, as there no lone pair electrons it geometry in octahedral.
S = 16 = ns2 np4 nd0
Question 52.
CO2 and H2O both are triatomic molecule but their dipole moment values are different. Why?
Answer:
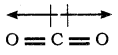
Sum of the dipole moment are cancelled.
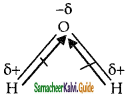
Water is ‘v’ shape sum of the dipole moments are not equal to zero.
Question 53.
Which one of the following has highest bond order?
(i) N2
(ii) N2+
(iii) N2–
Answer:
(i) N2
N2 = 14
σ1s2 σ1s*2 σ2s2 σ2s*2 π2py2 π2pz2 π2px2
Bond order = \(\frac{6}{2}\) = 3
(ii) N2+
σ1s2 σ1s*2 σ2s2 σ2s*2 π2py2 π2pz2 π2px1
Bond order = \(\frac{5}{2}\) = 2.5
(iii) N2–
σ1s2 σ1s*2 σ2s2 σ2s*2 π2py2 π2pz2 π2px*1
Bond order = \(\frac{5}{2}\) = 2.5
The highest bond order is N2
Question 54.
Explain the covalent character in ionic bond.
Answer:
Like the partial ionic character in covalent compounds, ionic compounds show partial covalent character. For example, the ionic compound, lithium chloride shows covalent character and is soluble in organic solvents such as ethanol.
The partial covalent character in ionic compounds can be explained on the basis of a phenomenon called polarisation. We know that in an ionic compound, there is an electrostatic attractive force between the cation and anion. The positively charged cation attracts the valence electrons of anion while repelling the nucleus.
This causes a distortion in the electron cloud of the anion and its electron density shift towards the cation, which results in some sharing of the valence electrons between these ions. Thus, a partial covalent character is developed between them. This phenomenon is called polarisation.
The ability of a cation to polarise an anion is called its polarising ability and the tendency of an anion to get polarised is called its polarisability.
![]()
Question 55.
Describe Fajan’s rule.
Answer:
(i) To show greater covalent character, both the cation and anion should have high charge on them. Higher the positive charge on the cation, greater will be the attraction on the electron cloud of the anion. Similarly higher the magnitude of negative charge on the anion, greater is its polarisabiity. Hence, the increase in charge on cation or in anion increases the covalent character.
Let us consider three ionic compounds aluminum chloride, magnesium chloride and sodium chloride. Since the charge of the cation increase in the order Na+ < Mg2+ <Al3+ the covalent character also follows the same order NaCl < MgCl2 < AlCl3.
(ii) The smaller cation and larger anion show greater covalent character due to the greater extent of polarisation. Lithium chloride is more covalent than sodium chloride. The size of Li+ is smaller than Na+ and hence the polarizing power of Li+ is more. Lithium iodide is more covalent than lithium chloride as the size of I– is larger than the Cl–. Hence I– will be more polarized than Cl– by the cation, Li+.
(iii) Cations having ns2 np6 nd10 configuration show greater polarizing power than the cations with ns2 np6 configuration. Hence, they show greater covalent character. CuCl is more covalent than NaCl. Compared to Na+ (1.13 Å). Cu+(0.6 Å) is small and have 3s2 3p6 3d10 configuration.
Electronic configuration of Cu+: [Ar] 3s2, 3p6, 3d10
Electronic Configuration of Na+: [He] 2s2, 2p6.
11th Chemistry Guide Chemical Bonding Additional Questions and Answers
I. Choose the best answer:
Question 1.
Which among the following elements has the tendency to form covalent compounds?
a) Ba
b) Be
c) Mg
d) Ca
Answer:
b) Be
Question 2.
The valency of C in CO32- is
a) 2
b) 3
c) 4
d) -3
Answer:
c) 4
Question 3.
Two elements X and Y have following electronic configurations
X = 1s2 2s2 2p6 3s2 3p6 4s2
Y = 1s2 2s2 2p6 3s2 3p5
The expressed compound formed by combination of X and Y will be expressed as
a) X2
b) X5Y2
c) X2Y5
d) XY5
Answer:
a) X2
Question 4.
Lattice energy of an ionic compound depends upon:
a) Charge on the ions only
b) Size of the ions only
c) Packing of the ions only
d) Charge and size of the ion
Answer:
d) Charge and size of the ion
Question 5.
If the cyanide ion, the formal negative charge is on
a) C
b) N
c) Both C and N
d) Resonate between C and N
Answer:
b) N
![]()
Question 6.
The formal charge of the O – atoms in the ion ![]() is
is
a) -2
b) + 1
c) – 1
d) 0
Answer:
d) 0
Question 7.
A compound with the maximum ionic character is formed from
a) Na and F
b) Cs and F
c) Cs and I
d) Na and Cl
Answer:
b) Cs and F
Question 8.
Which of the following has the highest ionic character?
a) MgCl2
b) CaCl2
c) BaCl2
d) BeCl2
Answer:
c) BaCl2
Question 9.
Which of the following compounds has the maximum covalent nature?
a) LiCl
b) NaCl
c) KCl
d) CsCl
Answer:
a) LiCl
Question 10.
Among the following the maximum covalent character is shown by the compound
a) FeCl2
b) SnCl2
c) AlCl3
d) MgCl2
Answer:
c) AlCl3
![]()
Question 11.
Polarization is the distortion of the shape of an anion by an adjacently placed cation. Which of the following statements is correct?
a) maximum polarization is brought about by a cation of high charge
b) Minimum polarization is brought about by a cation of low radius
c) A large cation is likely to bring about a large degree of polarization
d) A small anion is likely to undergo a large degree of polarization
Answer:
a) maximum polarization is brought about by a cation of high charge
Question 12.
Which of the following Lewis structure does not contribute in resonance?
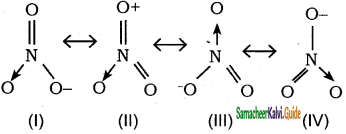
a) I
b) II
c) III
d) IV
Answer:
b) II
Question 13.
Point out incorrect statement about resonance
a) Resonance structures should have equal energy
b) In resonance structures, the constituent atoms should be in the same position
c) In resonance structures, there should not be the same number of electron pairs
d) Resonance structures should differ only in the location of electrons around the constituent atoms
Answer:
c) In resonance structures, there should not be the same number of electron pairs
Question 14.
A diatomic molecule has dipole moment of 1.2 D. If the bond distance is 1 Å. What percentage of electronic charge exists on each atom?
a) 12 % of e
b) 19 % of e
c) 25 % of e
d) 29 % of e
Answer:
c) 25 % of e
Question 15.
The electronegativity difference between two atoms A and B is 2, then percentage of covalent character in the molecule is
a) 54 %
b) 46 %
c) 23 %
d) 72 %
Answer:
a) 54 %
![]()
Question 16.
The electronegativity of H and Cl are 2.1 and 3.0 respectively. The correct statement (s) about the nature of HCl is/are:
a) 17 % ionic
b) 83 % ionic
c) 50 % ionic
d) 100 % ionic
Answer:
a) 17 % ionic
Question 17.
For the formation of covalent bond, the difference in the value of electronegativities should be
a) Equal to or less than 1.7
b) More than 1.7
c) 1.7 or more
d) None of these
Answer:
a) Equal to or less than 1.7
Question 18.
Pick out the molecule which has zero dipole moment
a) NH3
b) H2O
c) BCl3
d) SO2
Answer:
c) BCl3
Question 19.
The dipole moment of HBr is 1.6 × 10-30 cm and interatomic spacing is 1 Å. The % ionic character of HBr is
a) 7
b) 10
c) 15
d) 27
Answer:
b) 10
Question 20.
In a polar molecule, the ionic charge is 4.8 × 10-10 esu. If the inter ionic distance is 1 Å unit, then the dipole moment is
a) 41.8 debye
b) 4.18 debye
c) 4.8 debye
d) 0.48 debye
Answer:
c) 4.8 debye
![]()
Question 21.
Of the following molecules, the one, which has permanent dipole moment is:
a) SiF4
b) BF3
c) PF3
d) PF5
Answer:
c) PF3
Question 22.
Increasing order of dipole moment is:
a) CF4 < NH3 < NF3 < H2O
b) CF4 < NH3 < H2O < NF3
c) CF4 < NF3 < H2O < NH3
d) CF4 < NF3 < NH3 < H2O
Answer:
d) CF4 < NF3 < NH3 < H2O
Question 23.
The correct sequence of dipole moments among the chlorides of methane is
a) CHCl3 < CH2Cl2 > CH3Cl > CCl4
b) CH2Cl2 > CH3Cl > CHCl3 > CCl4
c) CH3Cl > CH2Cl2 > CHCl3 > CCl4
d) CH2Cl2 > CHCl3 > CH3Cl > CCl4
Answer:
c) CH3Cl > CH2Cl2 > CHCl3 > CCl4
Question 24.
Which of the following has been arranged in order of decreasing dipole moment?
a) CH3Cl > CH3F > CH3Br > CH3I
b) CH3F > CH3Cl > CH3Br > CH3I
c) CH3Cl > CH3Br > CH3I > CH3F
d) CH3F > CH3Cl > CH3I > CH3Br
Answer:
a) CH3Cl > CH3F > CH3Br > CH3I
Question 25.
Which of the following has the least dipole moment?
a) NF3
b) CO2
c) SO2
d) NH3
Answer:
b) CO2
![]()
Question 26.
Among the following compounds, the one that is polar and has central atom with sp2 hybridization is
a) H2CO3
b) SiF4
c) BF3
d) HClO2
Answer:
a) H2CO3
Question 27.
Which of the following will provide the most efficient overlap?
a) s – s
b) s – p
c) sp2 – sp2
d) sp – sp
Answer:
d) sp – sp
Question 28.
The number and type of bonds between two carbon atoms in CaC2 are:
a) one sigma (σ) and one pi (π) bonds
b) one sigma (σ) and two pi (π) bonds
c) one sigma (σ) and one half pi (π) bonds
d) one sigma (σ) bond
Answer:
b) one sigma (σ) and two pi (π) bonds
Question 29.
Which is not true according to VBT?
a) A covalent bond is formed by the overlapping of orbitals with unpaired electrons of opposite spins
b) A covalent bond is formed by the overlapping of orbitals with unpaired electrons of same spins
c) The greater the extent of overlapping the stronger is the bond
d) Overlapping takes place only in the direction of maximum electron density of the orbital
Answer:
b) A covalent bond is formed by the overlapping of orbitals with unpaired electrons of same spins
Question 30.
The hybridization of carbon atoms in C – C single bond of H – C ≡ C = CH = CH2 is
a) sp3 – sp3
b) sp2 – sp
c) sp – sp2
d) sp3 – sp
Answer:
b) sp2 – sp
![]()
Question 31.
The bond in the formation of fluorine molecule will be
a) Due to s – s overlapping
b) Due to s – p overlapping
c) Due to p – p overlapping
d) Due to hybridization
Answer:
c) Due to p – p overlapping
Question 32.
Which of the following overlaps gives a a bond with x as internuclear axis?
a) Pz and Pz
b) s and pz
c) s and Px
d) dx2 – y2 and dx2 – y2
Answer:
c) s and Px
Question 33.
The strength of bonds by overlapping of atomic orbitals is in the order
a) s – s > s – p > p – p
b) s – s < p – p < s – p
c) s – p < s – s < p – p
d) p – p < s – s < s – p
Answer:
a) s – s > s – p > p – p
Question 34.
Which cannot be explained by VBT?
a) Overlapping
b) Bond formation
c) Paramagnetic nature of oxygen
d) Shapes of molecules
Answer:
c) Paramagnetic nature of oxygen
Question 35.
The structure of IF7 is
a) square pyramidal
b) trigonal bipyramidal
c) octahedral
d) pentagonal bipyramidal
Answer:
d) pentagonal bipyramidal
![]()
Question 36.
The structure of XeOF4 is
a) tetrahedral
b) square pyramidal
c) square planner
d) octahedral
Answer:
b) square pyramidal
Question 37.
The molecule that has linear structure is :
a) CO2
b) NO2
c) SO2
d) SiO2
Answer:
a) CO2
Question 38.
The molecule which has pyramidal shape is:
a) PCl5
b) SO3
c) CO32-
d) NO3–
Answer:
a) PCl5
Question 39.
The type of hybrid orbitals used by the chlorine atom in ClO2–
a) sp3
b) sp2
c) sp
d) none of these
Answer:
a) sp3
Question 40.
Which one of the following molecule is planar?
a) NF3
b) NCl3
c) PH3
d) BF3
Answer:
d) BF3
![]()
Question 41.
Which one of the following compounds has sp2 hybridization?
a) CO2
b) SO2
c) NO2+
d) CO
Answer:
b) SO2
Question 42.
Which of the following molecules has trigonal planar geometry?
a) IF3
b) PCl3
c) NH3
d) BF3
Answer:
d) BF3
Question 43.
The percentage s – character of the hybrid orbitals in methane, ethane, and ethyne are respectively
a) 25, 33, 50
b) 25, 50, 75
c) 50, 75, 100
d) 10, 20, 40
Answer:
a) 25, 33, 50
Question 44.
Number of lone pair (s) in XeOF4 is / are
a) 0
b) 1
c) 2
d) 3
Answer:
b) 1
Question 45.
Which of the following molecule contains one pair of non – bonding electrons?
a) CH4
b) NH3
c) H2O
d) HF
Answer:
b) NH3
![]()
Question 46.
If XeF2, XeF4 and XeF6, the number of lone pair of electrons on Xe are respectively.
a) 2, 3, 1
b) 1, 2, 3
c) 4, 1, 2
d) 3, 2, 1
Answer:
d) 3, 2, 1
Question 47.
The shape of XeO2 F2 molecule is
a) Trigonal bipyramidal
b) square planar
c) tetrahedral
d) see – saw
Answer:
d) see – saw
Question 48.
According to MO theory,
a) O2+ is paramagnetic and bond order is greater than O2
b) O2+ is paramagnetic and bond order is less than O2
c) O2+ is diamagnetic and bond order is less than O2
d) O2+ is diamagnetic and bond order is more than O2
Answer:
a) O2+ is paramagnetic and bond order is greater than O2
Question 49.
Bond order of O2, O2+, O2– and O22- is in order
a) O2– < O22- < O2 < O2+
b) O22 < O2– < O2, < O2+
c) O2+ < O2 < O2– < O22-
d) O2, < O2+ < O2– < O22-
Answer:
b) O22 < O2– < O2, < O2+
Question 50.
Which of the following pairs have Identical values of bond order?
a) N2+ and O2+
b) F2 and Ne2
c) O2 and B2
d) C2 and N2
Answer:
a) N2+ and O2+
![]()
Question 51.
Which of the following is paramagnetic?
a) O2–
b) CN–
c) CO
d) NO+
Answer:
a) O2–
Question 52.
Which of the following compounds is paramagnetic?
a) CO
b) NO
c) O22-
d) O3
Answer:
b) NO
Question 53.
The number of antibonding electron pairs O22- molecular ion on the basis of molecular orbital theory is
a) 4
b) 3
c) 2
d) 5
Answer:
a) 4
Question 54.
The bond length of the species O2, O2+ and O2– if are in the order of
a) O2+ > O2 > O
b) O2+ > O2– > O2
c) O2 > O2+ > O2–
d) O2– > O2 > O2+
Answer:
a) O2+ > O2 > O
Question 55.
Which of the following is a zero overlap which leads to non – bonding?
a) 
b) ![]()
c) 
d) All
Answer:
a) 
![]()
Question 56.
Identify the least stable ion amongst the following:
a) Li–
b) Be–
c) B–
d) C–
Answer:
b) Be–
Question 57.
Which of the following molecular species has unpaired electrons(s)?
a) N2
b) F2
c) O2–
d) O22-
Answer:
c) O2–
Question 58.
Among the following species which has minimum bond length?
a) B2
b) C2
c) F2
d) O2–
Answer:
c) F2
Question 59.
The correct order of bond strength is :
a) O2– < O2 < O2+ < O22-
b) O22- < O2– < O2 < O2+
c) O2– < O22- < O2 < O2+
d) O2+ < O2 < O2– < O22-
Answer:
b) O22- < O2– < O2 < O2+
Question 60.
The species having bond order different from that in CO is
a) NO–
b) NO+
c) CN–
d) N2
Answer:
a) NO–
![]()
Question 61.
Which one of the following species is diamagnetic in nature?
a) He2+
b) H2
c) H2+
d) H2–
Answer:
b) H2
Question 62.
Which of the following species exhibits the diamagnetic behavior?
a) O22-
b) O2+
c) O2
d) NO
Answer:
a) O22-
Question 63.
Which one of the following pairs of species have the same bond order?
a) CN– and NO+
b) CN– and CN+
c) O2– and CN–
d) NO+ and CN+
Answer:
b) CN– and CN+
Question 64.
Which one is the the electron deficient compound?
a) ICl
b) NH3
c) BCl3
d) PCl3
Answer:
c) BCl3
Question 65.
The number of electrons shared by each outermost shell of N2 is
a) 2
b) 3
c) 4
d) 5
Answer:
b) 3
![]()
Question 66.
Which of the following has zero dipole moment?
a) CH2Cl2
b) CH4
c) NH3
d) PH3
Answer:
b) CH4
Question 67.
Which of the following statement is not correct?
a) Hybridization is the mixing of atomic orbitals prior to their combining into molecular orbitals
b) sp2 hybrid orbitals are formed from two p atomic orbitals and one s atomic orbital.
c) d2sp3 hybrid orbitals are directed towards the corners of a regular octahedron
d) dsp3 hybrid orbitals are all at 90° to one another
Answer:
d) dsp3 hybrid orbitals are all at 90° to one another
Question 68.
Shape of XeF4 molecule is
a) linear
b) pyramidal
c) tetrahedral
d) square planar
Answer:
d) square planar
Question 69.
Which of the following compounds, the one having a linear structure is
a) NH2
b) CH4
c) C2H2
d) H2O
Answer:
c) C2H2
Question 70.
The isoelectronic pair is
a) Cl2, ICl2–
b) ICl2–, ClO2
c) IF2+, I3–
d) ClO2–, ClF2+
Answer:
d) ClO2–, ClF2+
![]()
Question 71.
The bond order is maximum in
a) O2
b) O2–
c) O2+
d) O22-
Answer:
c) O2+
Question 72.
Which of the following does not exist on the basis of molecular orbital theory?
a) H2+
b) He2+
c) He2
d) Li2
Answer:
c) He2
Question 73.
Which of the following species have maximum number of unpaired electrons?
a) O2
b) O2+
c) O2–
d) O22-
Answer:
a) O2
Question 74.
In a polar molecule, the ionic charge is 4.8 × 10-10 esu. If the inter ionic distance is 1 Å unit, then the dipole moment is
a) 41.8 debye
b) 4.18 debye
c) 4.8 debye
d) 0.48 debye
Answer:
c) 4.8 debye
Question 75.
The molecule of CO2 has 180° bond angle. It can be explained on the basis of
a) sp3 hybridisation
b) sp2 hybridisation
c) sp hybridisation
d) d2sp3 hybridisation
Answer:
c) sp hybridisation
![]()
Question 76.
Which of the following have both polar and non – polar bonds?
a) C2H6
b) NH4Cl
c) HCl
d) AlCl3
Answer:
b) NH4Cl
Question 77.
Blue vitriol has
a) Ionic bond
b) Coordinate bond
c) Hydrogen bond
d) All the above
Answer:
d) All the above
Question 78.
The number of ionic, covalent, and coordinate bond NH4Cl are respectively
a) 1, 3 and 1
b) 1, 3 and 2
c) 1, 2 and 3
d) 1, 1 and 3
Answer:
b) 1, 3 and 2
Question 79.
Which of the following does not contain a coordinate bond?
a) H3O+
b) BF4–
c) HF2–
d) NH4+
Answer:
c) HF2–
Question 80.
Maximum covalent character is associated with the compound
a) NaI
b) MgI2
c) AlCl3
d) AlI3
Answer:
d) AlI3
![]()
Question 81.
Amongst LiCl, RbCl, BeCl3, and MgCl2 the compounds with the greatest and the least ionic character, respectively, are
a) LiCl and RbCl
b) RbCl and BeCl2
c) RbCl and MgCl2
d) MgCl2 and BeCl2
Answer:
b) RbCl and BeCl2
Question 82.
LiF is least soluble among the fluorides of alkali metals, because
a) smaller size Li+ impart significant covalent character in LiF
b) the hydration energies of Li+ and F– are quite higher
c) lattice energy of LiF is quite higher due to the smaller size of Li+ and F–
d) LiF has strong polymeric network in solid
Answer:
c) lattice energy of LiF is quite higher due to the smaller size of Li+ and F–
Question 83.
The molecule which has zero dipole moment is
a) CH2Cl2
b) BF3
c) NF3
d) ClO2
Answer:
b) BF3
Question 84.
Carbon tetrachloride has no net dipole moment because of
a) Its planar structure
b) Its regular tetrahedral structure
c) similar sizes of carbon and chlorine atoms
d) Similar electron affinities of carbon and chlorine
Answer:
b) Its regular tetrahedral structure
Question 85.
Of the following compounds, which will have a zero dipole moment?
a) 1, 1 dichloroethylene
b) cis – 1,2 dichloroethylene
c) trans – 1,2 dichloroethylene
d) none of these
Answer:
c) trans – 1,2 dichloroethylene
![]()
Question 86.
Which contains both polar and non – polar bonds?
a) NH 4Cl
b) HCN
c) H2O2
d) CH4
Answer:
c) H2O2
Question 87.
In which of the following species, is the underlined carbon has sp3 hybridisation:
a) CH3COOH
b) CH3CH2OH
c) CH3COCH3
d) CH2 = CH – CH3
Answer:
b) CH3CH2OH
Question 88.
Ration of a and bonds is maximum in
a) naphthalene
b) tetracyano methane
c) enolic form of urea
d) equal
Answer:
c) enolic form of urea
Question 89.
HCN and HNC molecules have equal number of
a) lone pair of σ bonds
b) σ bonds and π bonds
c) π bonds and lone pairs
d) lone pairs, σ bonds, and π bonds
Answer:
d) lone pairs, σ bonds, and π bonds
Question 90.
Allyl cyanide has
a) 9 sigma bonds and 4 pi bonds
b) 9 sigma bonds, 3 pi bonds, and 1 lone pair
c) 8 sigma bonds and 5 pi bonds
d) 8 sigma bonds, 3 pi bonds
Answer:
b) 9 sigma bonds, 3 pi bonds, and 1 lone pair
![]()
Question 91.
Effective overlapping will be shown by:
a) \(\oplus \Theta+\oplus \Theta\)
b) \(\left(\frac{\oplus}{\Theta}\right)+\left(\frac{\Theta}{\oplus}\right)\)
c) \(\oplus \Theta+\Theta \oplus\)
d) All the above
Answer:
c) \(\oplus \Theta+\Theta \oplus\)
Question 92.
In which of the following pairs, the two species are not Isostructural?
a) CO32- and NO3–
b) PCl4+; and SiCl4
c) PF5 and BrF5
d) AlF63- and SF6
Answer:
c) PF5 and BrF5
Question 93.
The hybridization of orbitals of N atom in NO3–, NO3+ and NH4+ are respectively.
a) sp, sp2, sp3
b) sp2, sp, sp3
c) sp, sp3, sp2
d) sp2, sp3, sp
Answer:
b) sp2, sp, sp3
Question 94.
On hybridization of one s and one p -orbital we get:
a) two mutually perpendicular orbitals
b) two orbitals at 180°
c) four orbitals directed tetrahedrally
d) three orbitais in a plane
Answer:
b) two orbitals at 180°
Question 95.
A molecule XY2 contains two σ bonds, two π bonds, and one lone pair of electrons in the valence shell of X. The arrangement of lone pair, as well as bond pairs, is
a) Square pyramidal
b) Linear
c) Trigonal planar
d) Unpredictable
Answer:
c) Trigonal planar
![]()
Question 96.
The maximum number of 90° angles between bond pair of electron is observed in :
a) sp3d2 hybridisation
b) sp3d hybridisation
c) dsp2 hybridisation
d) dsp3 hybridisation
Answer:
a) sp3 d2 hybridisation
Question 97.
Which of the following has a 3 centred 2 electron bond?
a) BF3
b) NH3
c) CO2
d) B2H6
Answer:
d) B2H6
Question 98.
Which has regular tetrahedral geometry?
a) SF4
b) BF4–
c) XeF4
d) [Ni(CN)4]2-
Answer:
b) BF4–
Question 99.
Which of the following statement is incorrect?
a) During N2+ formation, one electron is removed from the bonding molecular orbital of N2.
b) During O2+ formation, one electron is removed from the anti-bonding molecular orbital of O2.
c) During O2– formation, one electron is added to the bonding molecular orbital of O2
d) During CN– formation, one electron is added to the bonding molecular orbital of CN
Answer:
c) During O2– formation, one electron is added to the bonding molecular orbital of O2
Question 100.
Which concept best explains that O – nitrophenol is more volatile than p – nitrophenol?
a) Resonance
b) Hyper conjugation
c) Hydrogen bonding
d) Steric hindrance
Answer:
c) Hydrogen bonding
![]()
II. Very short question and answers (2 Marks):
Question 1.
State Octet rule.
Answer:
“The atoms transfer or share electrons so that all atoms involved in chemical bonding obtain 8 electrons in their outer shell (valence shell)”.
Question 2.
What is an electrovalent bond?
Answer:
The complete transfer of electron leads to the formation of a cation and an anion. Both these ions are held together by the electrostatic attractive force which is known as ionic bond.
Question 3.
What is covalent bond?
Answer:
This type of mutual sharing of one or more pairs of electrons between two combining atoms results in the formation of a chemical bond called a covalent bond
Question 4.
What is Co-ordinate bond?
Answer:
One of the combining atoms donates a pair of electrons i.e., two electrons which are necessary for the covalent bond formation, and these electrons are shared by both the combining atoms. These type of bonds are called coordinate covalent bond or coordinate bond.
Question 5.
Draw the lewis dot structure of the following.
(i) SO3
(ii) NH3
(iii) N2O5
Answer:
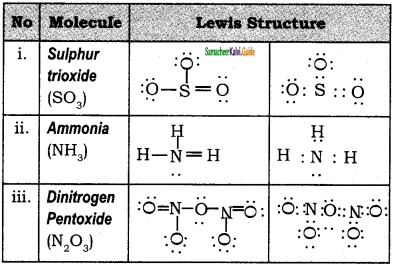
![]()
Question 6.
What is bond length?
Answer:
The distance between the nuclei of the two covalently bonded atoms is called bond length.
Example:
Carbon-carbon single bond length (1.54 Å).
Question 7.
What is bond angle?
Answer:
Covalent bonds are directional in nature and are oriented in specific directions in space. This directional nature creates a fixed angle between two covalent bonds in a molecule and this angle is termed as bond angle.
Example :
Molecule = CH4;
Atoms defining the angle = H-C-H
Bond angle is 109° 28′
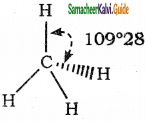
Question 8.
What is resonance?
Answer:
When we write Lewis structures for a molecule, more than one valid Lewis structures are possible in certain cases.
They only differ in the position of bonding and lone pair of electrons. Such structures are called structures (canonical structures) and this phenomenon is called resonance.
Question 9.
Draw the resonance structure of CO32- ion?
Answer:
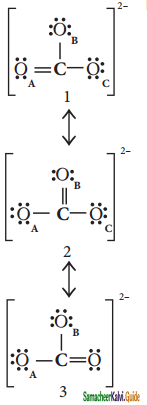
![]()
III. Short question and answers (3 Marks):
Question 1.
What is dipole moment of a covalent bond in a polar molecule?
Answer:
Dipole moment of a covalent bond in a polar molecule is defined as the product of the magnitude of the charge present on either of the two atoms and the distance by which the two atoms are separated in the molecule. It is a vector quantity and works in the direction of the line joining the positively charged end to the negatively charged end.
The unit of dipole moment is Debye.
Thus 1D = 1 × 10-18 e.s.u.cm.
Question 2.
Distinguish between σ – molecular orbital & π – molecular orbital.
Answer:
| σ molecular orbital | π molecular orbital |
| 1. It is formed by head on overlapping of atomic orbitals. | It is formed by the sidewise overlapping of atomic orbitals. |
| 2. The overlapping of atomic orbital is maximum. | The overlapping of atomic orbital is less. |
| 3. The orbital is symmetrical to rotation about the intemuclear axis. | The orbital is not symmetrical to rotation about the internuclear axis. |
| 4. The resulting covalent bond is strong. | The resulting covalent bond is weak. |
Question 3.
Explain formation of H2 molecule by MO theory.
Answer:
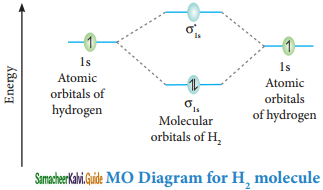
Molecular orbital diagram of hydrogen molecule (H2)
Electronic configuration of H atom 1s1
Electronic configuration of H2 molecule: σ1s2
Bond order = \(\frac{N_{b}-N_{a}}{2}=\frac{2-0}{2}\) = 1
Molecule has no impaired electrons hence it is dimagnetic.
Question 4.
Explain the formation of Li2 molecule by MOT.
Answer:
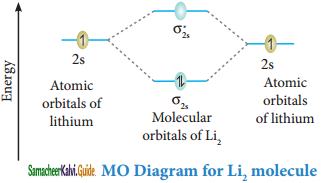
Molecular orbital diagram of Lithium molecule (Li2)
Electronic configuration of Li atom 1s2 2s1
Electronic configuration of Li2 molecule: σ1s2 σ1s*2 σ2s2
Bond order = \(\frac{N_{b}-N_{a}}{2}=\frac{4-2}{2}\) = 1
Molecule has no impaired electrons hence it is dimagnetic.
Question 5.
Explain the formation of B2 molecule by MOT.
Answer:
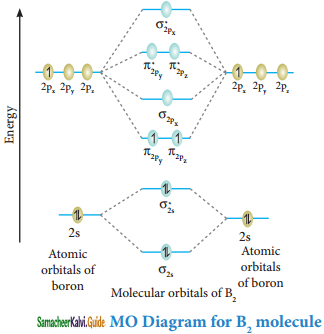
Molecular orbital diagram of Boron molecule (B2)
Electronic configuration of B atom 1s2 2s2 2P1
Electronic configuration of B2 molecule: σ1s2 σ1s*2 σ2s2 σ2s*2 π2py1 π2pz1
Bond order = \(\frac{N_{b}-N_{a}}{2}=\frac{6-4}{2}\) = 1
Molecule has two unpaired electrons hence it is paramagnetic.
![]()
Question 6.
Explain the formation of C2 molecule by MOT.
Answer:
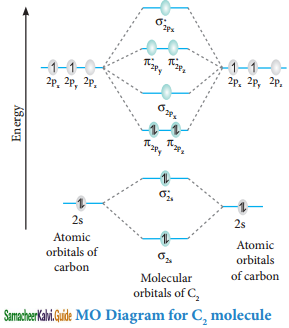
Molecular orbital diagram of Carbon molecule (B2)
Electronic configuration of C atom 1s2 2s2 2p1
Electronic configuration of C2 molecule: σ1s2 σ1s*2 σ2s2 σ2s*2 π2py1 π2pz1
Bond order = \(\frac{N_{b}-N_{a}}{2}=\frac{8-4}{2}\) = 2
Molecule has two unpaired electrons hence it is diamagnetic.
Question 7.
How is σ and π – bond formed?
Answer:
When two atomic orbitals overlap linearly along the axis, the resultant bond is called a sigma (σ) bond. This overlap is also called ‘Tread – on overlap” or “axial overlap”. Overlap involves an orbital (s- s and s – p overlaps) will always result in a sigma bond as the s orbital is spherical. Overlap between two p orbitals along the molecular axis will also result in sigma bond formation. When we consider x-axis as molecular axis, the px – px overlap will result in σ – bond.
When two atomic orbitals overlaps sideways, the resultant covalent bond is called a pi bond. When we consider x – axis as molecular axis, py – py and pz – pz overlap will result in the formation of a π – bond.
Question 8.
Explain Salient features of VB theory?
Answer:
Salient features :
- When half filled orbitais of two atoms overlap, a covalent bond will be formed between them.
- The resultant overlapping orbital is occupied by the two electrons with opposite spins. For example, when H2 is formed, the two is electrons of two hydrogen atoms get paired up and occupy the overlapped orbital.
- The strength of a covalent bond depends upon the extent of overlap of atomic orbitals. Greater the overlap, larger is the energy released and stronger will be the bond formed.
- Each atomic orbital has a specific direction (except s-orbital which is spherical) and hence orbital overlap takes place in the direction that maximizes overlap.
- Let us explain the covalent bond formation in hydrogen, fluorine, and hydrogen fluoride using VB theory.
Question 9.
How is HF & F2 molecule formed by using VB theory?
Answer:
Formation of HF molecule:
1. Electronic configuration of hydrogen atom is 1s1
2. Valence shell electronic configuration of fluorine atom: 2s2, 2px2, 2py2, 2pz1
3. When half filled 1s orbital of hydrogen linearly overlaps with a half filled 2pz orbital of fluorine, a σ – covalent bond is formed between hydrogen and fluorine.
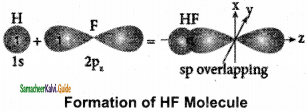
Formation of fluorine molecule (F2):
1. Valence shell electronic configuration of fluorine atom: 2s2, 2px2, 2Py2 2pz1
2. When the half filled pz orbitals of two fluorine overlaps along the z-axis, a σ – covalent bond is formed between them.
![]()
IV. Long question and answers (5 Marks):
Question 1.
Explain the Postulates of VSEPR theory.
Answer:
- The shape of the molecule depends on the number of valence shell electron pair around the central atom.
- There are two types of electron pairs namely bond pairs and lone pairs. The bond pair of electrons are those shared between two atoms, while the lone pairs are the valence electron pairs that are not involved in bonding.
- Each pair of valence electrons around the central atom repels each other and hence, they are located as far away as possible in three dimensional space to minimize the repulsion between them.
- The repulsive interaction between the different types of electron pairs is in the following order.
1p – 1p > 1p – bp > bp – bp
1p – lone pair; bp – bond pair - The lone pair of electrons are localised only on the central atom and interacts with only one nucleus whereas the bond pairs are shared between two atoms and they interact with two nuclei.
- Because of this lone pairs occupy more space and have greater repulsive power than the bond pairs in a molecule.
Question 2.
Explain the qualitative treatment of VB theory for the formation of H2 molecule.
Answer:
A simple qualitative treatment of VB theory for the formation of hydrogen molecule is discussed below.
Consider a situation where in two hydrogen atoms (Ha and Hb) are separated by infinite distance. At this stage there is no interaction between these two atoms and the potential energy of this system is arbitrarily taken as zero. As these two atoms approach each other, in addition to the electrostatic attractive force between the nucleus and its own electron (purple arrows), the following new forces begins to operate.
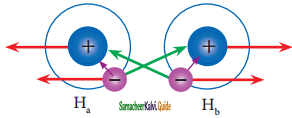
VB theory for the formation of hydrogen molecule
The new attractive forces (green arrows) arise between
(i) nucleus of Ha and valence electron of Hb
(ii) nucleus of Hb and the valence electron of Ha.
The new repulsive forces (red arrows) arise between
(i) nucleus of Ha and Hb
(ii) valence electrons of Ha and Hb
The attractive forces tend to bring Ha and Hb together whereas the repulsive forces tends to push them apart. At the initial stage, as the two hydrogen atoms approach each other, the attractive forces are stronger than the repulsive forces and the potential energy decreases. A stage is reached where the net attractive forces are exactly balanced by repulsive forces and the potential energy of the system acquires a minimum energy.
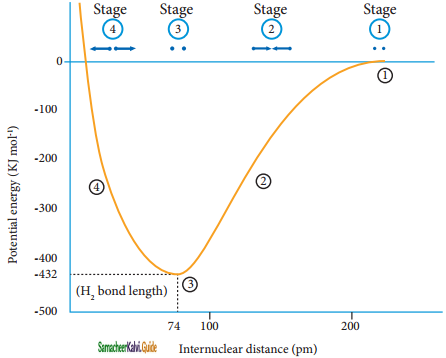
At this stage, there is a maximum overlap between the atomic orbitals of Ha and Hb, and the atoms Ha and Hb are now said to be bonded together by a covalent bond. The internuclear distance at this stage gives the H – H bond length and is equal to 74 pm.
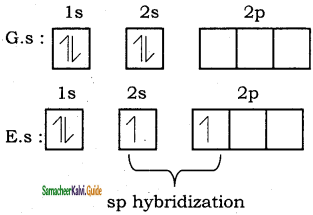
The liberated energy is 436 kJ mol-1 and is known as bond energy. Since the energy is released during the bond formation, the resultant molecule is more stable. If the distance between the two atoms is decreased further, the repulsive forces dominate the attractive forces and the potential energy of the system sharply increases.
![]()
Question 3.
Explain hybridization and geometry of BeCl2 molecule.
Answer:
Let us consider the bond formation in beryllium chloride. The valence shell electronic configuration of beryllium in the ground state is shown in the figure.
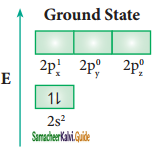

In BeCl2 both the Be-Cl bonds are equivalent and it was observed that the molecule is linear. VB theory explain this observed behaviour by sp hybridisation. One of the paired electrons in the 2s orbital gets excited to 2p orbital and the electronic configuration at the excited state is shown.
Now, the 2s and 2p orbitals hybridise and produce two equivalent sp hybridised orbitals which have 50% s-character and 50% p-character. These sp hybridised orbitals are oriented in opposite direction as shown in the figure.
Overlap with orbital of chlorine:
Each of the sp hybridized orbitals linearly overlap with pz orbital of the chlorine to form a covalent bond between Be and Cl as shown in the Figure

sp hybridization of BeCl2
Question 4.
Distinguish between σ – bond and π – bond.
Answer:
| Sigma (σ) Bond | Pi (π) Bond |
| 1. Sigma (σ) bond is formed by axial overlap of atomic orbitals. | Pi (π) bond is formed by the sidewise overlap of atomic orbitals. |
| 2. This bond can be formed by overlap of s-s, s-p or p-p orbitals. | It involves of overlap of p-p orbitals only. |
| 3. The bond is strong because, overlapping can take place to a large extent. | The bond is weak because the overlapping occurs to a small extent. |
| 4. The electron cloud formed by axial overlap is symmetrical about the inter nuclear axis and consists of single charged cloud. | The electron cloud of Pi bond is discontinuous and consists of two changed clouds above and below the plane of atoms. |
| 5. There can be a free rotation of atoms about the σ bond. | Free rotation of atoms around π bond is not possible because it involves breaking of π -bond. |
| 6. The bond may be present between the two atoms either alone or along with a π -bond. | The bond is always present between the two atoms along with the sigma (σ) bond. |
| 7. The shape of molecule is determined by the sigma framework around the central atom. | The π bonds do not contribute to the shape. |
![]()
Question 5.
Explain hybridisation & geometry of methane molecule?
Answer:
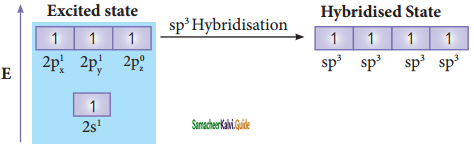
sp3 hybridisation can be explained by considering methane as an example. In methane molecule the central carbon atom bound to four hydrogen atoms. The ground state valence shell electronic configuration of carbon is [He]2s2 2px1 2py1 2Pz0.
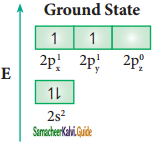
In order to form four covalent bonds with the four hydrogen atoms, one of the paired electrons in the 2s orbital of
carbon is promoted to its 2pz orbital in the excite state. The one 2s orbital and the three 2p orbitals of carbon mixes to give four equivalent sp3 hybridised orbitals. The angle between any two sp3 hybridised orbitals is 109° 28′.
Overlap with 1s orbitals of hydrogen:
The 1s orbitals of the four hydrogen atoms overlap linearly with the four sp3 hybridised orbitals of carbon to form four C-H σ-bonds in the methane molecule, as shown below.
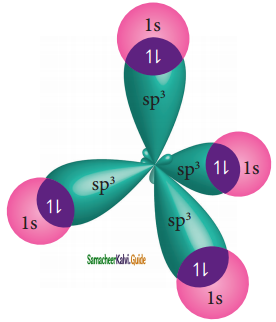
sp3 hybridization of CH4
Question 6.
Explain hybridization & geometry of PCl5 molecule.
Answer:
In the molecules such as PCl5, the central atom phosphorus is covalently bound to five chlorine atoms. Here the atomic orbitals of phosphorous undergoes sp3d hybridization which involves its one 3s orbital, three 3p orbitals and one vacant 3d orbital (dz2). The ground state electronic configuration of phosphorous is [Ne] 3s2 3px2 3py1 3pz1 as shown below.

One of the paired electrons in the 3s orbital of phosphorous is promoted to one of its vacant 3d orbital (dz2) in the excite state. One 3s orbital, three 3p orbitals, and one 3dz2. orbital of phosphorus atom mixes to give five equivalent sp3d hybridised orbitals. The orbitals geometry of sp3d hyrbridised orbitals is trigonal bi – pyramidal.
Overlap with 3pz orbitals of chlorine :
The 3pz orbitals of the five chlorine atoms linearly overlap along the axis with the five sp3d hybridized orbitals of phosphorous to form the five P – Cl σ – bonds, as shown below.
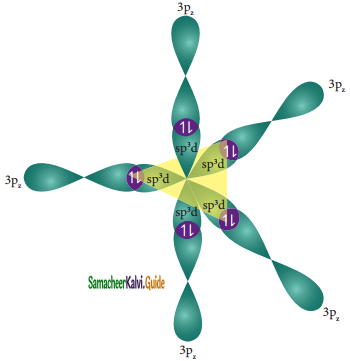
![]()
Question 7.
Explain hybridization & geometry of SF6 molecule.
Answer:
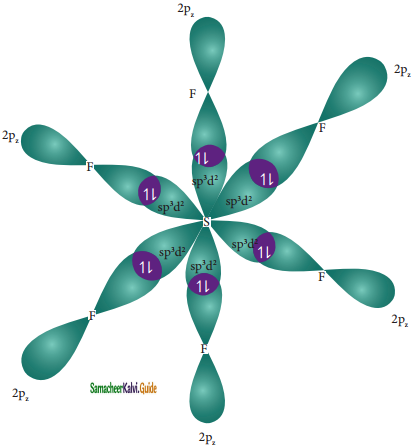
In sulphur hexafluoride (SF6) the central atom sulphur extend its octet to undergo sp3d2 hybridization to generate six sp3d2 hybridized orbitals which accounts for six equivalent S – F bonds. The ground state electronic configuration of sulphur is [Ne] 3s2 3px2, 3py2, 3pz1
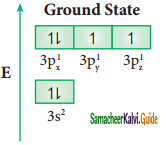
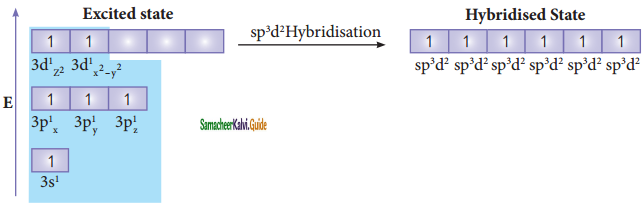
One electron each from 3s orbital and 3p orbital of sulphur is promoted to its two vacant 3d orbitals (dz2 and dx2 – y2) in the excite state. A total of six valence orbitals from sulphur (one 3s orbital, three 3p orbitals, and two 3d orbitals) mixes to give six equivalent sp3d2 hybridized orbitals. The orbital geometry is octahedral as shown in the figure.
Overlap with 2pz orbitals of fluorine:
The six sp3d2 hybridized orbitals of sulphur overlaps linearly with 2pz orbitals of six fluorine atoms to form the six S – F bonds in the sulphur hexafluoride molecule.
Question 8.
Write the postulates of molecular orbital theory.
Answer:
- When atoms combines to form molecules, their individual atomic orbitals lose their identity and forms new orbitals called molecule orbitals.
- The shapes of molecular orbitals depend upon the shapes of combining atomic orbitals.
- The number of molecular orbitals formed is the same as the number of combining atomic orbitals. Half the number of molecular orbitals formed will have lower energy than the corresponding atomic orbital, while the remaining molecular orbitals will have higher energy.
- The molecular orbital with lower energy is called bonding molecular orbital and the one with higher energy is called anti-bonding molecular orbital. The bonding molecular orbitals are represented as σ (Sigma), π (pi), δ (delta) and the corresponding antibonding orbitals are denoted as σ*, π* and δ*.
- The electrons in a molecule are accommodated in the newly formed molecular orbitals. The filling of electrons in these orbitals follows Aufbau’s principle, Pauli’s exclusion principle, and Hund’s rule as in the case of filling of electrons in atomic orbitals.
- Bond order gives the number of covalent bonds between the two combining atoms. The bond order of a molecule can be calculated using the following equation
- Bond order = \(\frac{\mathrm{N}_{\mathrm{b}}-\mathrm{N}_{\mathrm{a}}}{2}\)
Where, Nb = Total number of electrons present in the bonding molecular orbitals.
Na = Total number of electrons present in the antibonding molecular orbitals.
A bond order of zero value indicates that the molecule doesn’t exist.
Question 9.
Distinguish between molecular orbital & anti bonding molecular orbitals.
Answer:
| Bonding molecular orbital | Anti-bonding molecular orbital |
| 1. A bonding molecular orbital is formed when the electron waves of combining atoms are in phase, i.e the lobes of atomic orbitals have same sign. | An anti-bonding molecular orbital is formed when the electron waves of the combining atoms are not in phase ie the lobes of atomic orbital have opposite sign. |
| 2. For bonding molecular orbital wave function are summed up. | For anti-bonding molecular orbital, the wave functions are subtracted. |
| 3. The electron density is centered between the nuclei of the combining atoms. | The probability of finding the electron between the nuclei of the combining atom is negligible. |
| 4. The energy of bonding molecular orbital is less than that of the atomic orbitals of the combining atoms. | The energy of an antibonding molecular orbital is higher than the atomic orbitals of combining atoms. |
| 5. Electron present in bonding molecular orbital lead to attraction between the atoms and stabilize the molecule. | Electrons present in anti-bonding molecular orbitals lead to repulsion between the atoms and destabilize the molecule. |
![]()
Question 10.
Explain the formation of NO by MOT?
Answer:
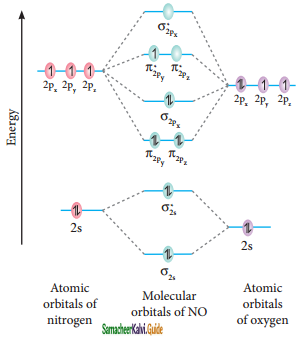
Molecular orbital diagram of nitrogen molecule (NO)
Electronic configuration of N atom 1s2 2s2 2p3
Electronic configuration of NO molecule
σ1s2 σ1s*2 σ2s2 σ2s*2 π2py2 π2pz2 σ2px2 π2py*1
Bond order = \(\frac{N_{b}-N_{a}}{2}=\frac{10-5}{2}\) = 2.5
Molecule has one unpaired electrons hence it is paramagnetic.